Surgical Insights for ODsIn the December 2024 issue of Review of Optometry—the magazine's 31st annual surgery report—learn how to spot and manage cataract surgery complications, review the newest techniques and procedures in refractive surgery and discover how laser procedures like SLT, LPI and YAG capsulotomy help your patients in the long term. Check out the other articles featured in this issue: |
Refractive surgery is a constantly evolving field, with new techniques and technologies emerging at a rapid pace. As primary eyecare providers, it’s crucial to stay up to date with these advancements to provide the best possible care and guidance to our patients. In this article, I’ll explore some of the latest developments in refractive surgery, from topography-guided LASIK to SMILE and more. Additionally, I’ll offer a glimpse into the future of refractive surgery, highlighting the exciting advancements currently being developed.
Topography-Guided LASIK: Personalized Precision
Traditional LASIK surgery revolutionized vision correction by reshaping the cornea using excimer laser technology rather than a scalpel. However, it primarily focused on correcting the overall curvature of the anterior cornea, while leaving behind subtle imperfections on the corneal surface that are known to cause higher-order aberrations (HOAs). These aberrations could lead to visual disturbances like glare, halos or reduced contrast sensitivity, particularly in low-light conditions.
The advent of wavefront-guided LASIK marked a significant leap forward. By using advanced technology to create a detailed map of the eye’s unique optical characteristics, surgeons can now tailor the laser treatment to address not only the major refractive errors but also pre-existing corneal irregularities that contribute to complex astigmatic patterns. This personalized approach led to improved visual outcomes, with many patients experiencing sharper vision and reduced night-vision disturbances.
Building upon this foundation, topography-guided LASIK represents the next frontier in LASIK technology. In addition to the wavefront data, this technique incorporates a high-resolution 3D mapping of the cornea’s surface. This allows surgeons to identify and correct even more subtle irregularities on the corneal surface, even at the time of the surgery, further enhancing visual acuity (VA) and reducing the risk of postoperative visual complications.
Topography-guided LASIK differs from traditional and wavefront-guided LASIK in the following ways:
 |
|
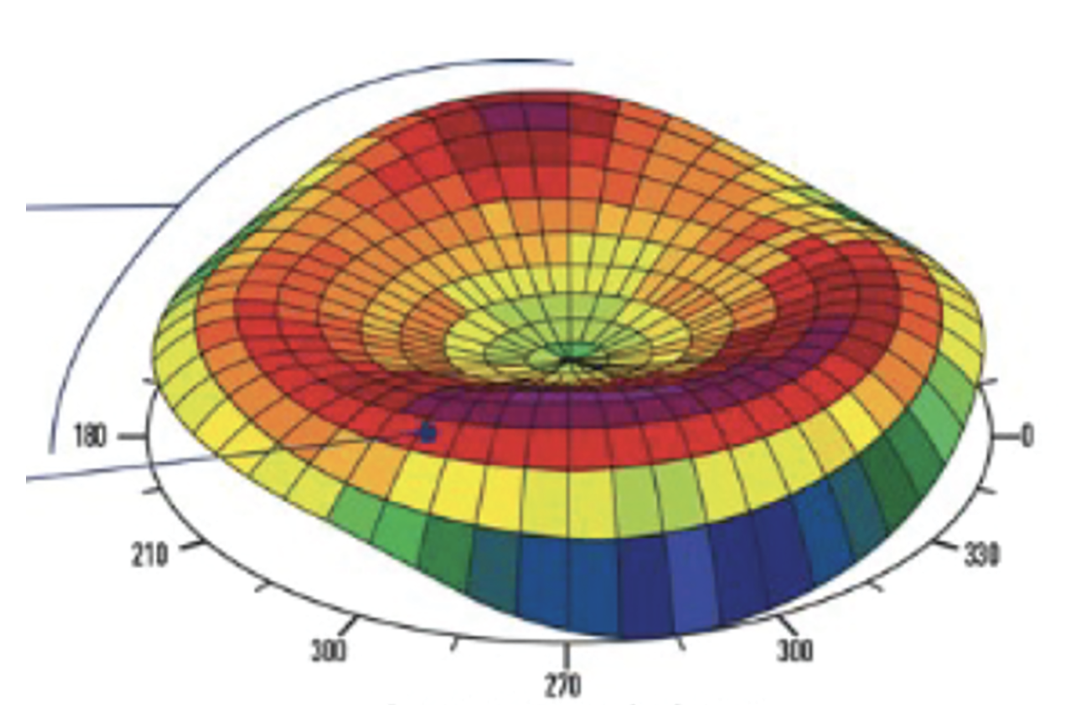
Fig. 1. Contoura Vision’s advanced corneal mapping technology captures 22,000 unique elevation points per eye, creating detailed 3D topographic maps for customized LASIK treatments. Photo: Alcon. Click image to enlarge. |
Customization. Traditional LASIK employs a standard ablation pattern based on the patient’s refractive error. Conversely, topography-guided LASIK uses detailed corneal mapping to create a personalized ablation profile that addresses both lower-order aberrations and HOAs unique to each eye (Figure 1).¹ This 3D mapping of the corneal surface allows for precise measurement of the curvature, irregularities and other characteristics of the cornea. By analyzing this data, the surgeon can identify and address specific areas of irregularities on the corneal surface that may be affecting vision quality.¹
Wavefront-guided LASIK uses a wavefront map that shows how light travels through the entire eye, including the cornea, lens and other structures. This map is like a detailed blueprint of the eye’s optical system, revealing not only the shape of the cornea but also any irregularities or distortions in how light passes through the eye. It has been demonstrated that topography-guided LASIK resulted in a greater reduction of HOAs compared to wavefront-guided LASIK.²
Precision. The ability to analyze the fine intricacies of the corneal surface at both a quantitative and qualitative level has ushered in a new era for topo-guided LASIK. This advancement allows for enhanced accuracy in reshaping the cornea by precisely pinpointing areas of irregularity.¹ It translates to improved visual outcomes, particularly in low-light settings where glare and halos are minimized.2-6 Research has also shown that topo-guided LASIK results in superior contrast sensitivity and reduced night vision symptoms compared to wavefront-optimized LASIK.³ The personalized approach of topo-guided LASIK minimizes risk of overcorrection or undercorrection, promoting better long-term stability and reducing the need for enhancement procedures.⁴ Due to its ability to address subtle corneal irregularities more effectively, topo-guided LASIK typically results in fewer retreatments than wavefront-optimized LASIK.⁴
Vision outcomes. Especially for patients with significant HOAs, topo-guided LASIK offers several potential benefits in terms of vision:
- Improved VA: Topography-guided LASIK can help patients achieve sharper vision and enhanced contrast sensitivity, particularly those with HOAs that affect visual quality beyond lower-order aberrations such as nearsightedness or farsightedness.3
- Reduced glare and halos: Topography-guided LASIK demonstrates superior night vision outcomes compared to wavefront-guided procedures, primarily due to its more conservative tissue ablation approach. By removing less stromal tissue and maintaining more of the cornea’s natural shape, this technique minimizes induced HOAs that typically cause night vision disturbances.4
- Enhanced visual quality: Patients often report a subjective improvement in overall visual quality after topography-guided LASIK, including reduced eye strain, clearer night vision and richer color perception.5
- Undercorrection/overcorrection: Topo-guided LASIK can help minimize the risk of undercorrection or overcorrection, which can occur when the ablation pattern is not perfectly aligned with the cornea’s irregularities. This can result in better long-term visual outcomes and reduce the need for additional procedures.4
One thing topography-guided and wavefront-guided LASIK have in common is their excellent safety profiles. Both techniques present minimal risk of significant complications, and studies have shown no significant difference in the incidence of major adverse events or vision-threatening complications between the two.5 Both procedures also have minor side effects, resulting in dry eye or temporary visual disturbances that typically resolve within a few weeks postoperatively.5
 |
|
Click image to enlarge. |
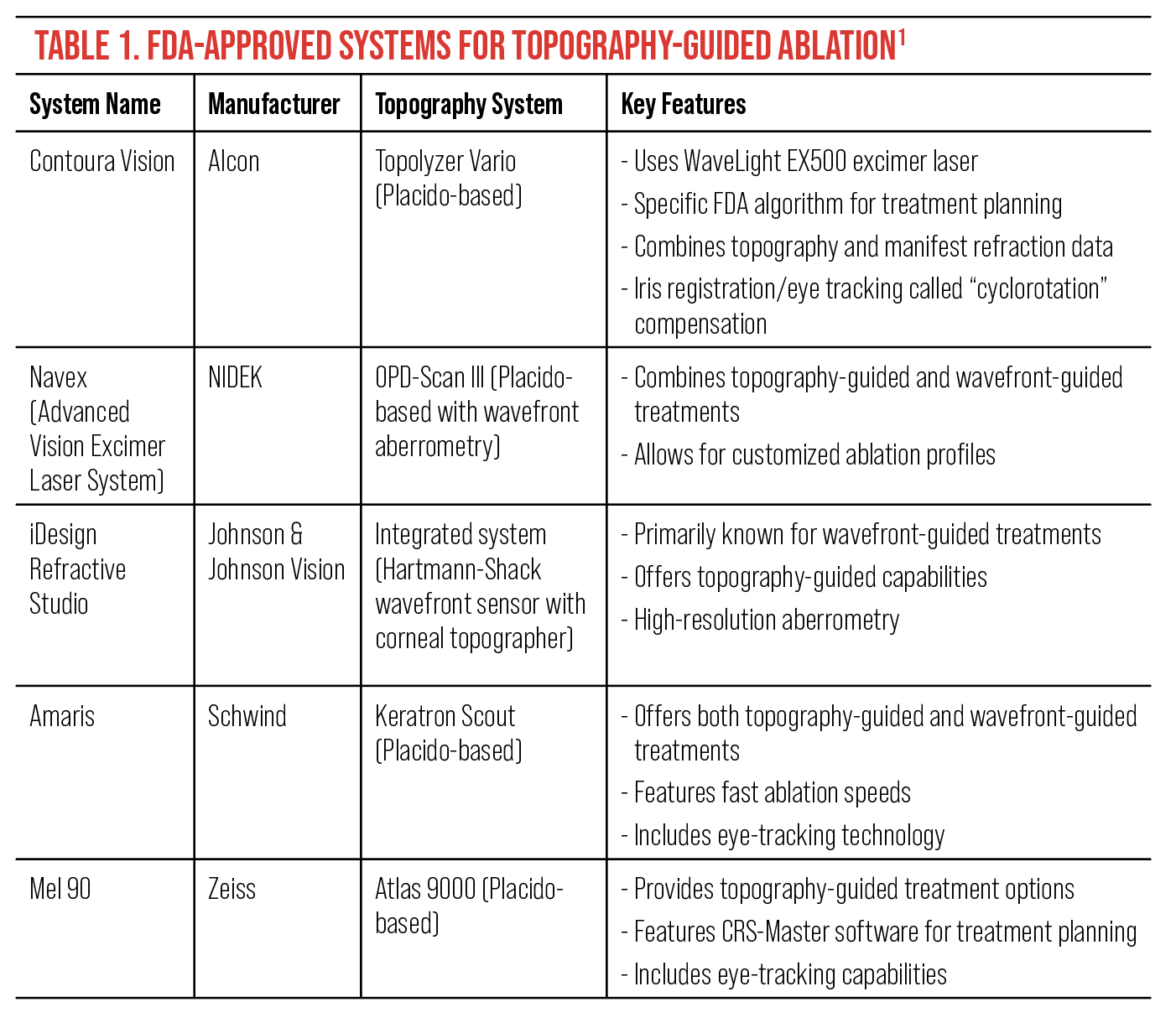
These systems approved by the FDA typically have specific criteria required to ensure optimal treatment outcomes (Table 1).6 While exact parameters may vary between laser platforms, the general principles remain consistent across systems. These criteria often include:
Astigmatism axis alignment: For patients with higher degrees of astigmatism (typically ≥2.00D), the difference in the astigmatic axis between manifest refraction and topographic data should be minimal, often not exceeding 5° to 10°.
Lower astigmatism considerations: In these cases (generally <2.00D astigmatism), a slightly larger discrepancy between manifest and topographic astigmatic axes may be acceptable, often up to 10° to 15°.
Astigmatic power consistency: The difference in astigmatic power between manifest refraction and topographic data should be relatively small, typically not exceeding 0.75D to 1.00D.
In essence, topography-guided LASIK has evolved beyond simply reshaping the cornea; it now also refines its surface texture. This meticulous customization often results in a more natural visual experience, with many patients reporting vision surpassing what they achieved with glasses or contact lenses.1
The evolution of LASIK from traditional to wavefront-guided and now topography-guided demonstrates the ongoing pursuit of precision and personalization in vision correction. As technology continues to advance, we can anticipate even more refined techniques that will further improve visual outcomes and expand the possibilities for patients seeking optimal vision.
SMILE: Minimally Invasive Vision Correction
Small incision lenticule extraction (SMILE) is the newest FDA-approved refractive laser surgery for common refractive errors (Figures 2 and 3).⁷ At present, candidacy for SMILE requires patients to have myopia within the range of -1.00D to -10.00D. The procedure can also treat astigmatism up to -3.00D within the FDA-approved parameters.⁸ This procedure has many key differences that make it unique. It is a completely different way of performing refractive surgery than the current methods. Only one laser is used during the entire process, unlike the various forms of LASIK.⁹
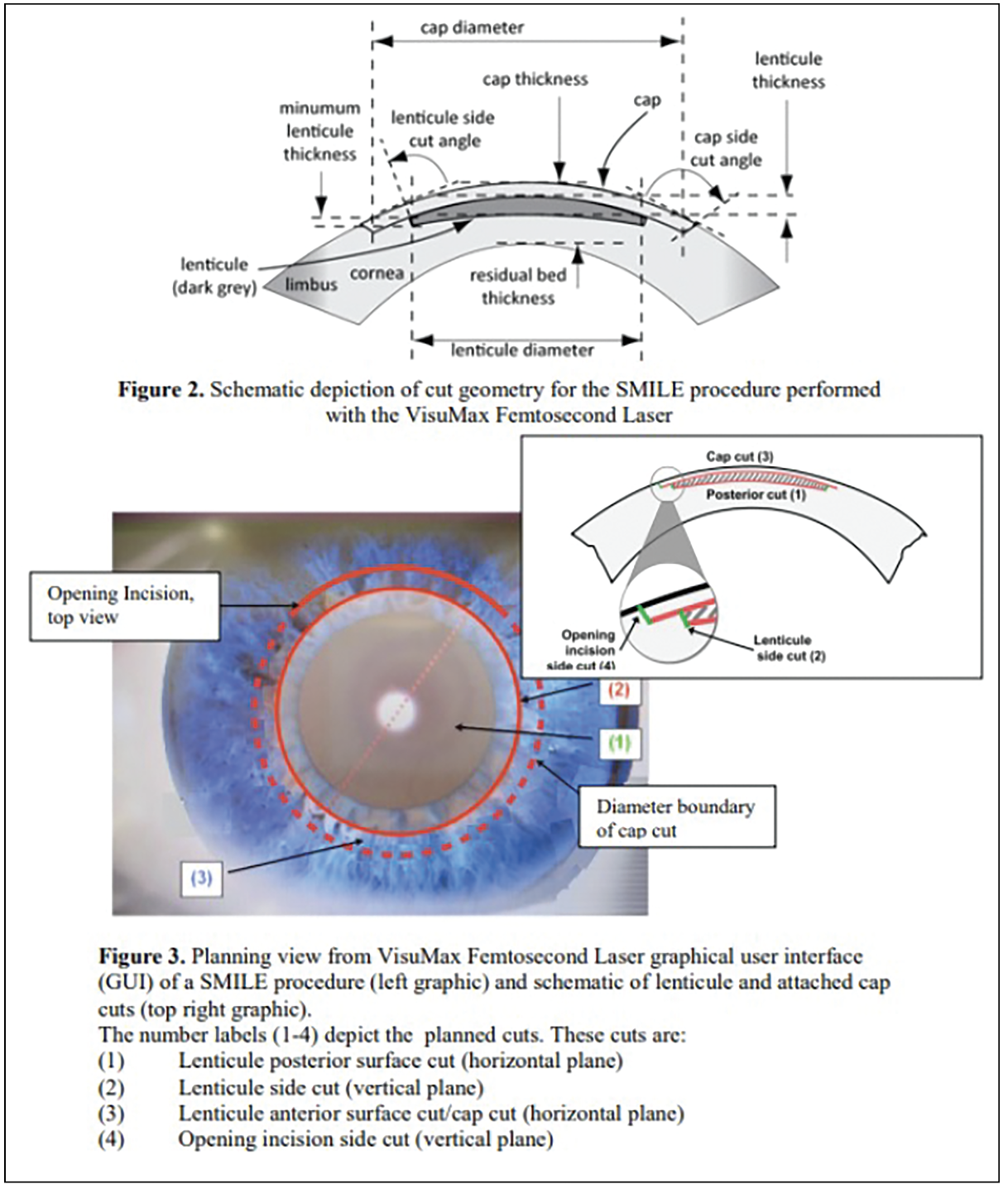 |
| Figs. 2 and 3. These images illustrate the key components of the SMILE procedure; the top diagram shows the schematic cross-section of cut geometry, detailing the lenticule dimensions and corneal parameters. The lower image displays the VisuMax Femtosecond Laser graphical user interface during surgery, showing the planned circular incision pattern and the four sequential laser cuts required to create and extract the lenticule. Photo: Carl Zeiss Meditec. Click image to enlarge. |
During the SMILE procedure, a femtosecond laser (VisuMax, Carl Zeiss Meditec) is used to create a small, arc-shaped incision on the corneal surface. The same laser then creates a thin disc of corneal tissue, called a lenticule, within the cornea stroma. The surgeon then carefully removes the lenticule through the small incision, reshaping the cornea and correcting the refractive error.
Because the incision made in SMILE is significantly smaller than the flap created in LASIK, there are many benefits, including better corneal biomechanics. However, potential drawbacks include a higher cost compared to LASIK, a narrower range of treatable refractive errors, a slightly longer recovery time, an increased risk of night aberrations and more limited enhancement options.9
SMILE has become an established form of refractive surgery as of late in the US and has been for even longer worldwide. Boasting excellent visual outcomes with a good safety profile, this procedure is a viable treatment option for myopia and myopic astigmatism in the absence of any corneal surface disorder.
Let’s review some of the unique features of SMILE:
Minimally invasive. SMILE involves a much smaller incision than LASIK, reducing the risk of complications and potentially improving healing time. Initially, the lenticule was extracted by lifting an epithelial flap similar to LASIK, then evolved to a single clear peripheral 2mm corneal incision.⁸
Intrastromal. SMILE’s intrastromal approach, where the lenticule is created and extracted within the cornea, preserves the integrity of the anterior stroma, the cornea’s strongest layer. With a cap thickness fixed at 120µm, SMILE maintains most of the biomechanically important anterior stromal tissue. Research shows the posterior 60% of the stroma is 50% weaker than the anterior 40% of the corneal stroma.7,11 This translates to enhanced biomechanical stability, potentially reducing the risk of post-surgical ectasia and promoting faster visual recovery.¹² Furthermore, by sparing the anterior stroma, SMILE minimizes the chance of damaging corneal nerve fibers believed to be associated with postoperative dry eye.¹⁰,¹³
All-in-one. SMILE offers a significant advantage in surgical efficiency by using a single femtosecond laser for both the incision and lenticule creation. This streamlined approach eliminates the need for an excimer laser, effectively optimizing operating room space and resource allocation.⁹
The Future of SMILE
Recent research suggests promising developments in SMILE technology, with ongoing studies exploring its potential applications beyond current indications. While the procedure has shown encouraging results for treating hyperopia in early studies, its expansion into broader refractive corrections awaits further clinical validation and regulatory approval. The emergence of new variants demonstrates the continued evolution of this technology, pointing to future refinements in refractive surgery techniques.
While SMILE may offer a safe treatment option for a broader range of hyperopia compared to LASIK, it is important to note that it currently lacks FDA approval for this specific indication. Due to its relative novelty, long-term studies are scarce. However, a promising prospective study involving 93 eyes treated with SMILE for hyperopia demonstrated encouraging results, with 95% achieving uncorrected VA of 20/40 or better and maintaining good corneal stability after 12 months.¹⁴ These results suggest that SMILE could be a viable treatment for hyperopia in the future, pending further research and regulatory approval. In Europe, SMILE is currently being performed on up to +6.00D of hyperopia and 5.00D of cylinder.7
A new SMILE variant, SILK (smooth incision lenticule keratomileusis, uses a different femtosecond laser that creates a smaller lenticule.15 While not yet FDA-approved, SILK offers promising advancements as a future modification of the SMILE procedure.
The core of SILK is the Elita femtosecond laser (Johnson & Johnson Vision), operating at an unprecedented 10MHz level compared to the kilohertz levels of existing systems. This high-speed operation combined with low-energy settings (<50nJ) results in exceptionally smooth lenticule creation. Key features include real-time adjustment for pupil centration, cyclotorsion control and overlapping spots for nearly dissection-free lenticule removal.
The Elita laser’s capacity for real-time adjustments based on pupil centration and cyclo-rotation is particularly noteworthy. This could enhance refractive outcomes and streamline lenticule removal, potentially leading to more predictable results and shorter surgical times.
As SILK progresses through clinical trials and regulatory approval processes, its role in refractive surgery practices remains to be determined. Rigorous clinical studies will be crucial to fully understand its long-term efficacy, safety profile and potential advantages over established refractive procedures.
PRK: The Comeback Kid
 |
|
Fig. 4. Many of the newer PRK techniques reduce risk of postoperative corneal haze formation. Photo: Christopher J. Rapuano, MD. Click image to enlarge. |
Photorefractive keratectomy (PRK), once overshadowed by LASIK, is experiencing a resurgence in popularity. This renaissance is driven by several factors, including improved surgical techniques, advanced laser technology and a growing recognition of its benefits for certain patients. Below, we will highlight some of these recent innovations and the advantages of PRK.
Transepithelial PRK (T-PRK). Traditional PRK, while effective, involves the burden of requiring manual or alcohol-assisted removal of the corneal epithelium before laser ablation. This step, though necessary, could lead to potential complications and discomfort. Alternatively, T-PRK has revolutionized this process by using the excimer laser to precisely ablate the epithelium, making the procedure less invasive and potentially more comfortable for patients.
Some advantages of T-PRK include:
- Reduced complication risk: Eliminating manual or chemical epithelial removal minimizes the risk of infection, corneal haze (scarring), postoperative discomfort, dry eye and other complications associated with traditional/alcohol-assisted PRK (Figure 4).¹⁶
- More uniform ablation surface: The laser creates a smoother, more uniform ablation surface, which can lead to better visual outcomes and reduced risk of irregular astigmatism.¹⁷
- Potentially faster visual recovery: Due to much less re-epithelialization required from T-PRK, patients often return to normal activities sooner and have better VA in the early postoperative period compared to traditional PRK.16,17
Topography-guided PRK. This represents a significant advancement in bringing customizable refractive surgery to a broader patient population. This variant of PRK has emerged as a significant refinement in corneal refractive procedures, offering enhanced precision in treating both regular ametropia and irregular astigmatism. Modern excimer laser platforms have evolved to support this advancement, featuring sophisticated eye-tracking systems that compensate for involuntary eye movements and ensure precise ablation delivery.
This technique uses high-resolution corneal topography data to generate a customized ablation profile, effectively addressing not only refractive errors but also corneal surface irregularities that may be undetectable or untreatable with traditional wavefront-guided or wavefront-optimized approaches. Enhanced ablation speeds in contemporary laser systems reduce corneal exposure time, potentially contributing to more predictable outcomes and faster healing.
The fundamental principle underlying topography-guided treatments is the integration of anterior corneal surface data with refractive error measurements to create a more comprehensive treatment plan. By combining wavefront- and topography-guided approaches, surgeons can develop personalized ablation profiles based on each patient’s unique corneal characteristics. This integrated approach allows for simultaneous correction of lower-order aberrations and regularization of the corneal surface, potentially mitigating HOAs associated with corneal irregularities.
Other prospects for PRK include:
Expanded candidacy. Due to its ability to treat a wider range of refractive errors and corneal conditions, PRK remains an attractive option for patients who are not suitable candidates for LASIK or other corneal refractive procedures. One example is patients with thinner corneas: by avoiding the creation of a corneal flap, PRK preserves more corneal tissue, making it a safer option for these individuals.
The long-term efficacy and safety of PRK in patients with thin corneas have been investigated with promising results. One study found PRK to be safe and effective in patients with corneas thinner than 500µm, noting stable refractive outcomes and no cases of ectasia over a 10-year follow-up period.18
PRK may also offer advantages for patients with pre-existing dry eye syndrome or those at higher risk of developing dry eye after refractive surgery. The procedure preserves more corneal nerves than LASIK, potentially resulting in less severe and shorter-duration dry eye symptoms postoperatively.
Research from 2015 comparing dry eye symptoms and tear film parameters after PRK and LASIK found that while both procedures induced dry eye symptoms initially, the PRK group showed faster recovery and better tear film stability at six months post-op.19
Reduced risk of flap-related complications. By eliminating the need for a corneal flap, PRK avoids potential flap-related complications associated with LASIK, such as flap dislocation, striae or epithelial ingrowth. This makes PRK an attractive option for patients with active lifestyles or those in professions with a higher risk of eye trauma.
Treatment of irregular astigmatism. PRK, especially when combined with topography-guided treatments, has shown significant efficacy in managing irregular astigmatism, a condition that can be challenging to treat with traditional refractive surgery techniques.
- Post-trauma or post-surgical irregularities: A 2020 study on the use of topography-guided PRK for treating irregular astigmatism following radial keratotomy demonstrated significant improvements in both uncorrected and corrected VA, as well as reductions in HOAs.20
- Keratoconus: In mild to moderate cases, topo-guided PRK combined with corneal crosslinking (the “Athens Protocol”) has shown promising results in keratoconus (more on this later). One study reported long-term stability and improved visual outcomes in keratoconic eyes treated with this approach.21
- Decentered ablations: For patients with decentered ablations from previous refractive surgeries, topography-guided PRK can help regularize the corneal surface. One study from 2018 demonstrated the efficacy of this approach in treating highly aberrated eyes with significant visual symptoms.22
Expanded range for hyperopia treatment. While PRK has traditionally been associated with myopic corrections, advancements in laser technology and treatment protocols have expanded its application to hyperopic treatments.
- Higher hyperopic corrections: Modern PRK techniques can effectively treat higher degrees of hyperopia compared to earlier iterations. Research from 2020 that evaluated outcomes of PRK for hyperopia up to +6.00D reported safe and effective results with good long-term stability.23
- Hyperopic astigmatism: PRK has shown efficacy in treating compound hyperopic astigmatism. A study reporting on the long-term outcomes of PRK for compound hyperopic astigmatism demonstrated stable refractive results and high patient satisfaction over a 10-year follow-up period.24
- Presbyopic hyperopes: For presbyopic patients with hyperopia, PRK can be combined with monovision or blended vision techniques. Recent research exploring the outcomes of hyperopic PRK with monovision reported high levels of spectacle independence and patient satisfaction.25
These expanded applications underscore PRK’s versatility in modern refractive surgery. By offering effective treatment options for irregular astigmatism and a wider range of hyperopic corrections, PRK continues to play a crucial role in addressing complex refractive cases and providing tailored solutions for patients who may not be candidates for other procedures.
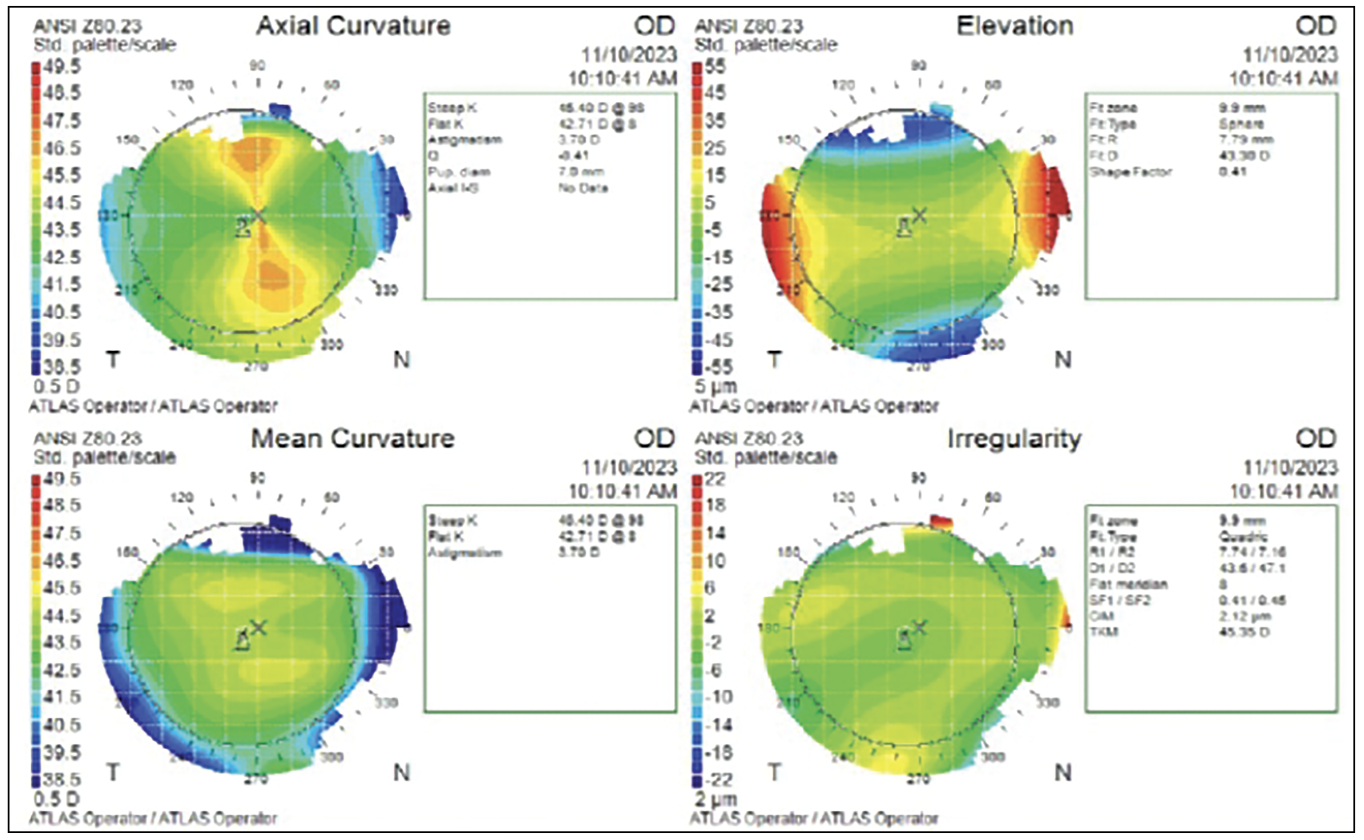 |
|
Fig. 5. Preoperative corneal topography of the right eye was obtained prior to PRK. The left eye (not pictured) won’t be operated on, as its inherent manifest refraction of -1.50D provides good near vision. To ensure patient adaptation to monovision, a contact lens trial was conducted before surgery simulating the anticipated post-op refractive outcome. Click image to enlarge. |
PRK vs. LASIK: Long-term Outcomes
Multiple studies have shown that the long-term outcomes of PRK are comparable to LASIK in terms of VA, refractive stability and patient satisfaction. A nine-year follow-up study from 2015 comparing long-term outcomes of LASIK and PRK for myopia found no significant difference in any of those three parameters between the two procedures.26 Another study from the same year examined the 20-year outcomes of PRK for myopia, reporting excellent long-term safety and efficacy, with stable refractive results and no significant long-term complications.27
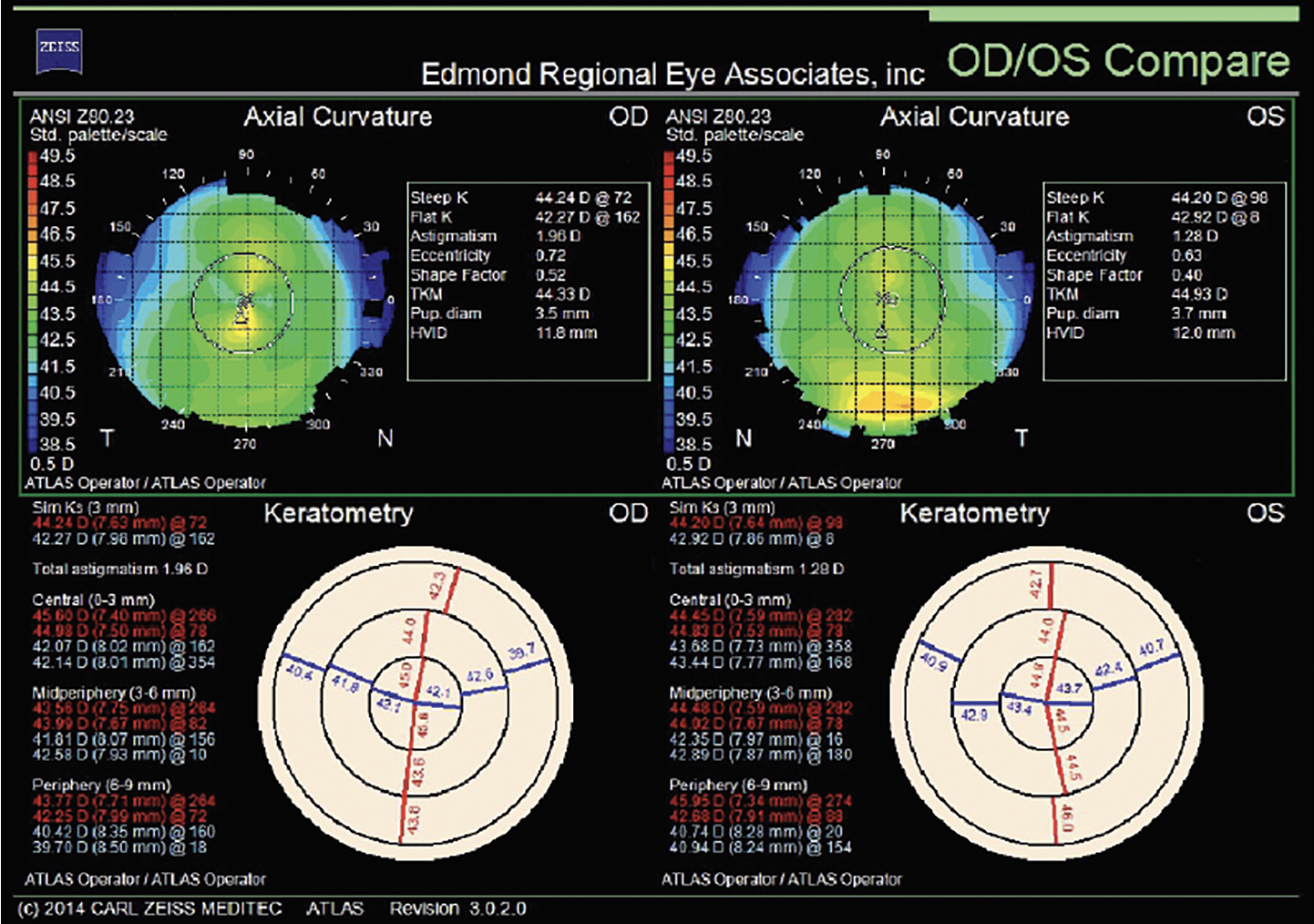 |
|
Fig. 6. Preoperative topographies OU before topography-guided PRK with a target of plano sphere OU. Click image to enlarge. |
Emerging Trends and Future Directions
As PRK continues to demonstrate excellent long-term outcomes comparable to LASIK, researchers and clinicians are exploring refinements and combinations to further enhance its safety and efficacy. Recent developments focus on optimizing treatment protocols, particularly through the integration of complementary therapies and techniques to address specific clinical challenges and expand treatment options for complex cases. Here are a few examples:
Combination therapies. The integration of PRK with complementary procedures has opened new avenues for treating complex corneal conditions and enhancing refractive outcomes. These combination approaches combine the strengths of multiple modalities to address a broader range of visual and structural corneal issues.
PRK with corneal crosslinking (CXL). The combination of PRK and CXL, often referred to as the “Athens Protocol” when performed simultaneously, has shown promising results in managing keratoconus and other corneal ectatic disorders.28-30
- Forme fruste keratoconus: The combination of PRK with accelerated CXL for patients with forme fruste keratoconus was recently demonstrated to improve visual outcomes and corneal stability compared to PRK alone.30
- Progressive keratoconus: A long-term study that evaluated the 10-year outcomes of topography-guided PRK combined with CXL for keratoconus reported stable visual and refractive outcomes, demonstrating the technique’s potential for managing progressive corneal ectasia.28,31
- Post-LASIK ectasia: Studies investigating the effectiveness of combined PRK with CXL in treating post-LASIK ectasia show significant improvements in corneal stability and visual outcomes. This approach has shown promise in halting ectasia progression while providing visual rehabilitation for patients with this challenging complication.32
PRK with phototherapeutic keratectomy (PTK). The combination of PRK and PTK has shown efficacy in treating corneal surface irregularities alongside refractive errors. This approach was recently evaluated in patients with corneal scarring and concurrent refractive errors, who showed significant improvements in both VA and corneal clarity.22
Emerging combination approaches. Several novel combination therapies involving PRK are currently under investigation:
- PRK with accelerated epithelial healing: In 2022, researchers began exploring the use of insulin-like growth factor-1 eye drops following PRK to accelerate epithelial healing and reduce postoperative discomfort.33
- PRK with autologous platelet-rich plasma: Early studies have shown potential benefits of using platelet-rich plasma in conjunction with PRK to enhance corneal wound healing and reduce haze.34
These combination therapies represent the cutting edge of refractive surgery, offering tailored approaches for complex cases that may not be suitable for standard procedures. As research progresses, we can expect further refinement of these techniques and the emergence of new combination approaches to address the diverse needs of our patients, potentially improving both visual and structural outcomes.
Takeaways
Refractive surgery is evolving at a remarkable pace, driven by groundbreaking research and technological innovations that could transform the lives of countless individuals by offering them the opportunity to achieve clear, spectacle-free vision and enhance their overall quality of life. Optometrists occupy a unique position at the forefront of patient care. Staying current on the latest refractive surgery techniques and technologies allows clinicians to guide patients toward the most appropriate and effective treatment options. As primary eyecare providers, it is our responsibility to educate patients about the risks and benefits of various procedures, ensuring they make informed decisions based on their needs and visual goals.
Furthermore, collaborating with refractive surgeons fosters a seamless continuum of care, enabling optometrists to provide comprehensive support throughout the patient journey. From pre-op evaluations and patient selection to post-op management and long-term follow-up, ODs play an integral role in optimizing patient outcomes and ensuring their visual well-being.
The future of refractive surgery is undoubtedly bright, with promising new technologies on the horizon. Optometry’s practice scope is expanding in a growing number of states, which may even include performing certain refractive surgery procedures. By embracing these advancements, ODs can empower patients to achieve their vision goals and enjoy the freedom of clear and comfortable vision.
Dr. Daniel specializes in ocular disease and refractive surgery at Edmond Regional Eye Associates in Edmond, OK, having received advanced clinical training in the diagnosis and management of ocular disease. He is also certified in laser vision correction, anterior segment laser procedures and other minor surgical procedures. Dr. Daniel is a fellow of the American Academy of Optometry, as well as a diplomate of the American Board of Optometry. He has no financial disclosures.
1. Ong T, Tone S. Topography-guided ablation for corneal refractive surgery. Asia Pac J Ophthalmol. 2021;10(5):453-61. 2. Kanellopoulos AJ, Asimellis G. Topography-guided LASIK versus wavefront-guided LASIK for myopia and astigmatism: a prospective contralateral eye study. J Refract Surg. 2014;30(3):145-52. 3. Mai ELC, Chang CK, Lee CY, et al. Higher-order aberrations of topography-guided LASIK and wavefront-optimized LASIK in high- and low-myopic eyes: a non-randomized controlled trial. J Pers Med. 2023;13(3):399. 4. Jain AK, Malhotra C, Pasari A, et al. Outcomes of topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia in virgin eyes. J Cataract Refract Surg. 2016;42(9):1302-11. 5. Stulting RD, Fant BS; T-CAT Study Group, et al. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11-8. 6. Ipek SC, Utine CA. Topography-guided excimer laser ablation in refractive surgery. Front Ophthalmol. 2024;4:1367258. 7. FDA. VisuMax femtosecond laser small incision lenticule extraction (SMILE). www.accessdata.fda.gov/cdrh_docs/pdf15/P150040D.pdf. Accessed October 15, 2024. 8. Shah R. History and results; indications and contraindications of SMILE compared with LASIK. Asia Pac J Ophthalmol (Phila). 2019;8(5):371-6. 9. Moshirfar M, Somani SN, Patel BC. Small incision lenticule extraction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. www.ncbi.nlm.nih.gov/books/NBK549896. Accessed October 15, 2024. 10. Wong AHY, Cheung RKY, Kua WN, et al. Dry eyes after SMILE. Asia Pac J Ophthalmol (Phila). 2019;8(5):397-405. 11. Randleman JB, Dawson DG, Grossniklaus HE, et al. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24:S85-9. 12. Zarei-Ghanavati S, Hassanzadeh S, Ambrósio R Jr. Corneal ectasia after laser-assisted small-incision lenticule extraction: the case for an enhanced ectasia risk assessment. J Current Ophthalmol. 2022;34(3):357-63. 13. Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521-42. 14. Pradhan KR, Reinstein DZ, Carp GI, et al. Small incision lenticule extraction (SMILE) for hyperopia: 12-month refractive and visual outcomes. J Refract Surg. 2019;35:442-50. 15. Schallhorn JM, Seifert S, Schallhorn SC. SMILE, topography-guided LASIK and wavefront-guided LASIK: review of clinical outcomes in premarket approval FDA studies. J Refract Surg. 2019;35(11):690-8. 16. Gaber MM, Saif M, Elsaftawy HSE, Gouda AT. Comparative study of transepithelial versus alcohol-assisted photorefractive keratectomy. Delta J Ophthalmol. 2024;25(3):142-9. 17. Fattah MA, Antonios R, Arba Mosquera S, et al. Epithelial erosions and refractive results after single-step transepithelial photorefractive keratectomy and alcohol-assisted photorefractive keratectomy in myopic eyes: a comparative evaluation over 12 months. Cornea. 2018;37(1):45-52. 18. Sia RK, Ryan DS, Edwards JD, Stutzman RD, Bower KS. The U.S. Army surface ablation study: comparison of PRK, MMC-PRK and LASEK in moderate to high myopia. J Refract Surg. 2014;30(4):256-64. 19. Lee JB, Ryu CH, Kim J, et al. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(9):1326-31. 20. Ghoreishi M, Peyman A, Koosha N, et al. Topography-guided transepithelial photorefractive keratectomy to correct irregular astigmatism after radial keratotomy. J Curr Ophthalmol. 2020;32(3):233-41. 21. Kanellopoulos AJ, Binder PS. Ten-year outcomes of progressive keratoconus management with the Athens protocol (topography-guided partial-refraction PRK combined with CXL). J Refract Surg. 2021;37(8):545-53. 22. Reinstein DZ, Archer TJ, Dickeson ZI, Gobbe M. Transepithelial phototherapeutic keratectomy protocol for treating irregular astigmatism based on population epithelial thickness measurements by Artemis very high-frequency digital ultrasound. J Refract Surg. 2015;30(6):380-387. 23. Antonios R, Arba Mosquera S, Awwad ST. Hyperopic laser in situ keratomileusis: comparison of femtosecond laser and mechanical microkeratome flap creation. J Cataract Refract Surg. 2020;41(8):1602-9. 24. Roszkowska AM, De Grazia L, Meduri A, et al. Long-term results of excimer laser procedure to correct astigmatic refractive errors. Med Sci Monitor. 2013;19:927-33. 25. Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA. Outcomes of wavefront-guided laser in situ keratomileusis using a new-generation Hartmann-Shack aberrometer in patients with high myopia. J Cataract Refract Surg. 2022;41(9):1810-9. 26. Shalchi Z, O’Brart DP, McDonald RJ, et al. Eighteen-year follow-up of excimer laser photorefractive keratectomy. J Cataract Refract Surg. 2015;41(1):23-32. 27. Alio JL, Soria FA, Abbouda A, Peña-García P. Fifteen years follow-up of photorefractive keratectomy up to 10D of myopia: outcomes and analysis of the refractive regression. Br J Ophthalmol. 2016;100(5):626-32. 28. Kanellopoulos AJ. Ten-year outcomes of progressive keratoconus management with the Athens protocol (topography-guided partial-refraction PRK combined with CXL). J Refract Surg. 2019;35(8):478-83. 29. Kanellopoulos AJ. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J Refract Surg. 2009;25(9):S812-818. 30. Kymionis GD, Portaliou DM, Kounis GA, et al. Simultaneous topography-guided photorefractive keratectomy followed by corneal collagen cross-linking for keratoconus. Am J Ophthalmol. 2011;152(5):748-55. 31. Taneri S. Evaluation of epithelial remodeling with anterior segment OCT after PRK for post-LASIK ectasia. J Refract Surg. 2017;33(10):680-7. 32. Kanellopoulos AJ, Binder PS. Management of corneal ectasia after LASIK with combined, same-day, topography-guided partial transepithelial PRK and collagen cross-linking: the Athens protocol. J Refract Surg. 2011;27(5):323-31. 33. Yin J. Accelerated reepithelialization after PRK by insulin-like growth factor-1 eye drops. Transl Vis Sci Technol. 2022;11(4):32. 34. Alio JL, Rodriguez AE, WróbelDudzińska D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol. 2017;26(4):325-32. |


