 |
|
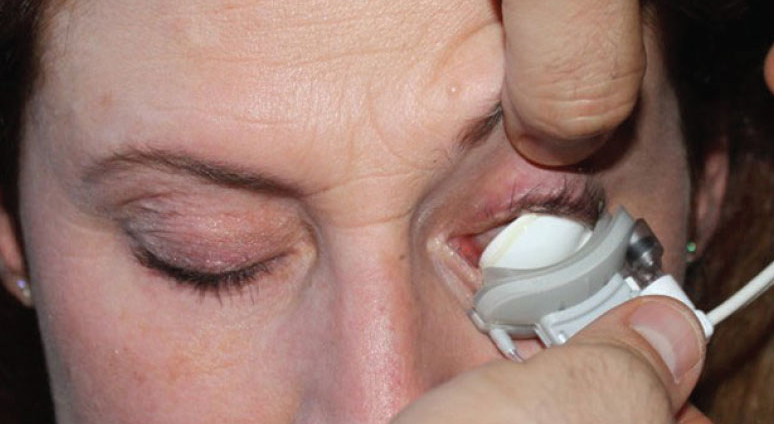
LipiFlow improved symptoms and meibomian gland function, even though outcomes were not significantly different from other dry eye disease treatments. Photo: Katherine Sanford, OD. |
Treating meibomian gland dysfunction (MGD) is critical to improving the lives of dry eye sufferers, but the many treatment options available can lead to “choice paralysis” among doctors. When warm compresses and eyelid hygiene are not enough, many turn to more advanced in-office treatments such as LipiFlow (Johnson & Johnson Vision). The unit consists of a desktop console that regulates the heat and pressure delivered to the patient’s eyelids via sterile single-use applicators that vault over the cornea. While convenient, it is often considered costly to the patient and the practice compared with warm compresses, especially given the fact that it is typically not covered by health care plans.
A team of researchers recently conducted the first Cochrane review to evaluate the effectiveness of LipiFlow for treating dry eye disease signs and symptoms and its safety compared with sham or other available treatments for MGD in adults. Their findings, which were published in Contact Lens and Anterior Eye, showed limited evidence of a clinically meaningful difference between LipiFlow and basic warm compresses, thermostatic devices, eyelid hygiene, topical dry eye disease medication or oral medications at the time points evaluated when the authors analyzed symptom scores based on questionnaires. Nevertheless, none of the 13 trials reported any intervention-related, vision-threatening adverse events.
“The available data suggest that LipiFlow performs similarly to other commonly used dry eye disease treatments regarding dry eye disease signs and symptoms,” the authors wrote in their study. “Of the trials reviewed, estimates of outcomes relating to symptoms, meibum expression score, quality of meibum and meibomian gland atrophy provided evidence of very low to low certainty.”
“While LipiFlow likely works for treating MGD, we were surprised to find that it performs similarly to other commonly used DED treatments,” says Andrew Pucker, executive director of clinical and medical sciences at Lexitas Pharma Services in Durham, NC, and coauthor of the study.
This study included 13 trials that randomized a total of 1,155 participants (66% female; age range = 19 to 86). Five trials compared LipiFlow with basic warm compresses, and another five compared LipiFlow with thermostatic devices. The remaining three included trials could not be grouped for comparisons.
Analyzing symptom scores in the first five trials yielded conflicting evidence of a difference in symptoms between LipiFlow and basic warm compresses after four weeks. There was no evidence of a difference in meibomian gland expression, meibum quality or tear breakup time when comparing LipiFlow with basic warm compresses. Analysis of symptom scores in the other five trials at four weeks showed that thermostatic devices had reduced Ocular Surface Disease Index (OSDI) scores by a mean difference of 4.59 as compared with LipiFlow.
“We were also surprised to find no studies with adequate masking and that testing methodology among the studies had a lot of variability, which made it challenging to analyze the collected data,” Dr. Pucker comments.
“Specifically, the included trials failed to consistently use the same questionnaire for evaluating dry eye disease symptoms, which made it challenging to discern whether there was a true between-interventions difference for our primary outcome,” the study noted.
Among other design weaknesses, studies often grouped treatments into different categories for comparison purposes. The research team noted that while grouping treatments by type was necessary for statistical comparisons, treatments within each group were not fully equivalent (e.g., LipiFlow, TearCare [Sight Science], iLux [Alcon] apply heat to the eyelid via different methods), and this should be considered when interpreting the results of this study.
Their review also highlighted the limited number of long-term randomized trials that have compared LipiFlow with other treatment options, which is problematic given that MGD is typically a chronic, lifelong condition. Only five of the 13 included trials reported findings longer than six months, and a single trial reported at 12 months.
“Due to a lack of comprehensive reporting of adverse events, the safety profile of LipiFlow in patients with MGD remains unclear, although none of the trials reported procedure-related sight-threatening complications,” the research team pointed out.
The summary of the Cochrane systematic review concluded that, without stronger evidence, providers may find it difficult to determine whether and when to recommend LipiFlow as a first-line or adjunctive treatment for meibomian gland dysfunction, especially given that LipiFlow often results in a high cost to the patient.
“We still need more high-quality studies that involve masking and a placebo control arm to fully understand the usefulness of LipiFlow for the treatment of MGD,” Dr. Pucker says.
| Click here for journal source. |
Yim TW, Pucker AD, Rueff E, et al. LipiFlow for the treatment of dry eye disease: a Cochrane systematic review summary. Cont Lens Anterior Eye. November 18, 2024. [Epub ahead of print]. |


