 |
Cystoid macular edema (CME) is a known complication, which has central visual consequences in individuals with retinitis pigmentosa (RP). Reduction in central visual acuity (VA) in addition to the peripheral visual field constriction and night vision challenges that individuals with RP experience can have a significant impact on quality of life and independence. While treatment options for CME in RP vary, the use of topical ophthalmic carbonic anhydrase inhibitors (CAIs) may provide visual benefit as a noninvasive, off-label therapy.
A 67-year-old man presented for evaluation of reduced vision, particularly in the right eye. He had history of RP, and while he doesn’t drive, he reported to begin to feel unsafe navigating due to constriction of his visual field in addition to the new change in central vision. He also had history of primary open-angle glaucoma in both eyes for which he used latanoprost 0.005% “a couple of times a week” in addition to generalized myasthenia gravis managed with pyridostigmine and oral prednisone, and hypercholesterolemia, managed with atorvastatin.
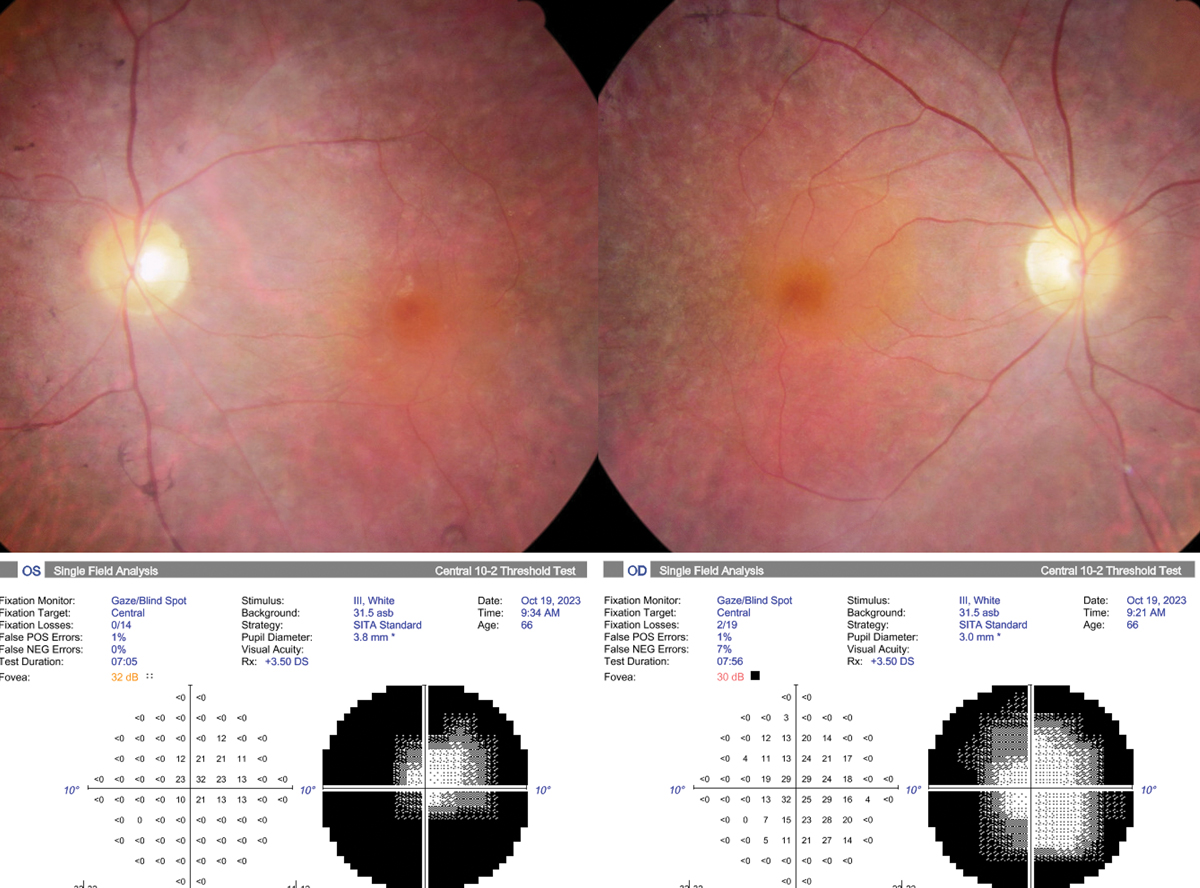
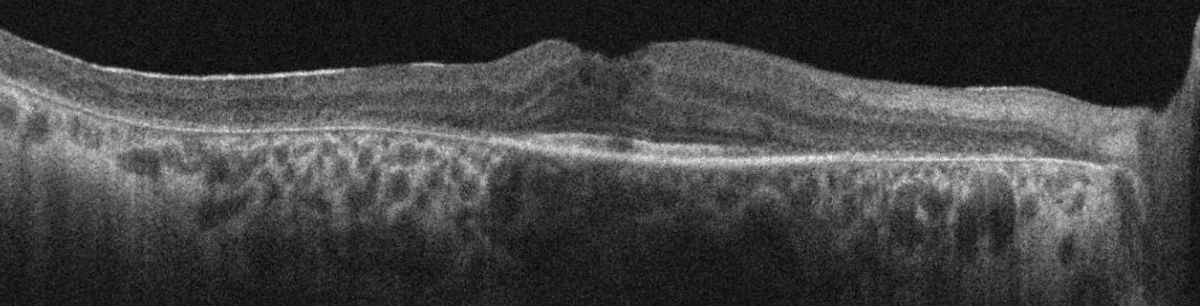
Best-corrected VA was 20/30- OD and OS and pupils were round and minimally reactive without afferent defect. Intraocular pressures (IOPs) were 12mm Hg in each eye, and he was pseudophakic bilaterally. Optic discs were sharp with 3+ diffuse pallor without notching of the neuroretinal rim. The macula appeared flat with epiretinal membrane bilaterally. There was attenuated retinal vasculature and diffuse 360° retinal pigmentary changes. Visual fields were severely constricted bilaterally and macular OCT demonstrated subtle cystoid macular edema along with parafoveal retinal pigment epithelium (RPE) and outer retinal atrophy with intact ellipsoid zone subfoveally and epiretinal membrane bilaterally.
 |
|
Fundus photograph and automated 10-2 visual field of the right and left eye. Click image to enlarge. |
CME and RP
This condition in the context of RP occurs in up to 50% of individuals as a result of a range of proposed mechanisms, including breakdown of the blood-retinal barrier, RPE pump dysfunction and inflammation.1,2 Treatment options range, in route of administration and treatment target, from oral or topical CAIs that target RPE pump function to intravitreal steroids aimed to reduce inflammatory markers and stabilize the outer blood-retinal barrier, as well as intravitreal anti-VEGF agents that decrease vascular permeability and restore compromised vascular endothelial function.1,3 Carbonic anhydrase inhibition in the treatment of RP-associated CME has been subject to the most attention in the literature, with 14 studies centered on safety and efficacy of 32 total studies in the treatment of RP-associated CME in a recent meta-analysis and systematic review.3
The utility of carbonic anhydrase inhibition in RP-associated CME is not a new concept, with initial publication of a prospective study in 1988.4 With the approval of topical ophthalmic dorzolamide 2% in 1994, considering the improved tolerability and safety profile vs. oral acetazolamide and similar response rate of approximately 40%, topical dorzolamide 2% three times daily is often positioned as a first-line off-label treatment in CME in the context of RP.3,5
 |
|
SD-OCT of the right macula demonstrating cystoid macular edema, epiretinal membrane and parafoveal ellipsoid zone loss. Click image to enlarge. |
Carbonic Anhydrase Inhibition
While we’re very familiar with CAIs and their role in reduction of IOP due to suppression of aqueous production, their role as an off-label treatment or adjunct in the management of conditions resulting in intraretinal or subretinal fluid accumulation have been explored considering their impact on the outer blood-retinal barrier (BRB) and RPE pump function.
Breakdown of the BRB, with the inner blood retinal barrier maintained by tight junctions between endothelial cells of retinal vasculature and the outer blood retinal barrier maintained by tight junctions of RPE cells leads to cystoid macular edema.6 Acetazolamide, whether orally or topically administered, inhibits carbonic anhydrase IV, present at both the apical and basal surface of RPE cells, increasing active transport through the outer BRB, increasing the net movement of fluid through the RPE towards the choroid and increasing RPE-retina adhesion.7-9
Potential risks related to topical CAI use should be evaluated prior to prescribing, including evaluation of presence of corneal endothelial dysfunction. Hypersensitivity to sulfonamide antibiotics is not an absolute contraindication to non-antibiotic sulfonamide use, including dorzolamide and acetazolamide. While the potential for cross-reactivity cannot be excluded, if an IgE-mediated allergy develops to a non-sulfonamide antibiotic such as dorzolamide in an individual with sulfonamide antibiotic allergy, it is most likely due to two separate allergies.10
 |
|
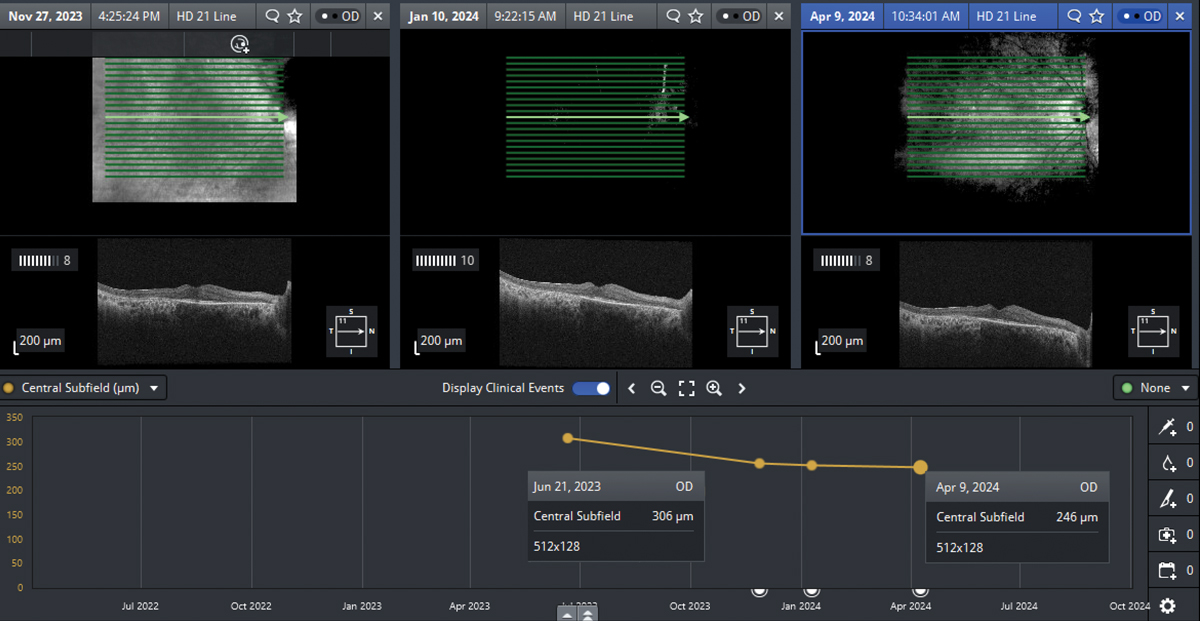
Reduction in central subfield thickness in the right eye following initiation of dorzolamide 2% three times daily. Click image to enlarge. |
The most common and expected ocular adverse effects of topical ophthalmic CAIs are burning, stinging and ocular discomfort associated with instillation, which were demonstrated in approximately 33% of patients in pivotal trials of dorzolamide 2%.5 Dorzolamide 2% has a pH of 5.6, while the pH of latanoprost 0.005% is 6.7, which impacts its overall tolerability upon instillation.5,11 Despite a similar pH to dorzolamide 2%, the fixed-dose combination of dorzolamide-timolol has been reported to have an improved tolerability profile with 21.5% of patients reporting ocular burning or stinging.12 While the fixed-dose combination of dorzolamide-timolol may improve tolerability vs. dorzolamide 2% alone, potential adverse effects of both dorzolamide and timolol should be carefully assessed prior to prescription. Topical dorzolamide is preferred to brinzolamide due to greater posterior segment tissue penetration as measured in animal models.13
Case Approach
After a discussion of risks, benefits, off-label use and expected effects, the patient was prescribed dorzolamide 2% three times daily in each eye with follow up examination set for six weeks. Despite the potential for improved tolerability, dorzolamide-timolol fixed combination was not used, as topical ophthalmic timolol is contraindicated due to the patient’s history of myasthenia gravis. He returned five months later with reported subjective improvement in VA in the right eye. Best-corrected VA was 20/25- OD and 20/30- OS, with a five-letter gain noted in the right eye. Central subfield thickness measured by OCT reduced from 306µm to 254µm in the right eye and has since remained stable, with a 23µm reduction in central subfield thickness observed in the left eye.
The patient’s optic disc appearance and visual field appearance were not consistent with glaucomatous optic neuropathy and latanoprost was discontinued without measurable change to central subfield thickness or IOP. Genetic testing was pursued at the patient’s request in consideration of his children with a variant of uncertain significance identified. Further evaluation was advised with a genetic counselor for a more detailed investigation. The patient continues to opt to use topical dorzolamide 2% two to three times daily bilaterally and receives periodic retinal evaluation in addition to low vision services.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Strong S, Liew G, Michaelides M. Retinitis pigmentosa-associated cystoid macular oedema: pathogenesis and avenues of intervention. Br J Ophthalmol. 2017;101(1):31-7. 2. Adackapara CA, Sunness JS, Dibernardo CW, et al. Prevalence of cystoid macular edema and stability in oct retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina. 2008;28(1):103-10. 3. Chen C, Liu X, Peng X. Management of cystoid macular edema in retinitis pigmentosa: a systematic review and meta-analysis. Front Med (Lausanne). 2022;16;9:895208. 4. Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190-5. 5. Merck & Co. Trusopt (dorzolamide ophthalmic solution) 2%. [package insert]. US Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2014/020408s050lbl.pdf. Revised December 2020. Accessed October 20, 2024. 6. Vinores SA. Breakdown of the blood–retinal barrier. Encyclopedia of the Eye. 2010:216-22. 7. Moldow B, Sander B, Larsen M, et al. The effect of acetazolamide on passive and active transport of fluorescein across the blood-retina barrier in retinitis pigmentosa complicated by macular oedema. Graefes Arch Clin Exp Ophthalmol. 1998;236(12):881-9. 8. Wolfensberger TJ, Mahieu I, Jarvis-Evans J, et al. Membrane-bound carbonic anhydrase in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35(9):3401-7. 9. Marmor MF. Control of subretinal fluid: experimental and clinical studies. Eye (Lond). 1990;4(Pt 2):340-4. 10. Guedes GB, Karan A, Mayer HR, Shields MB. Evaluation of adverse events in self-reported sulfa-allergic patients using topical carbonic anhydrase inhibitors. J Ocul Pharmacol Ther. 2013;29(5):456-61. 11. Pfizer. Xalatan (latanoprost ophthalmic solution) 0.005%. [package insert]. US Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020597s044lbl.pdf. Revised August 2011. Accessed October 20, 2024. 12. Shedden A, Adamsons IA, Getson AJ, et al. Comparison of the efficacy and tolerability of preservative-free and preservative-containing formulations of the dorzolamide/timolol fixed combination (COSOPT) in patients with elevated intraocular pressure in a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1757-64. 13. Kadam RS, Jadhav G, Ogidigben M, Kompella UB. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: implications for effects on ocular blood flow. Drug Metab Dispos. 2011;39(9):1529-37. |


