Clinical Research Crash CourseMedical statistics are only meaningful if you know how they’re calculated and why. To help ODs feel more confident interpreting clinical study findings and stats, Review of Optometry published a four-part series on scientific research and how it relates to clinical practice. Browse the other features in this series: |
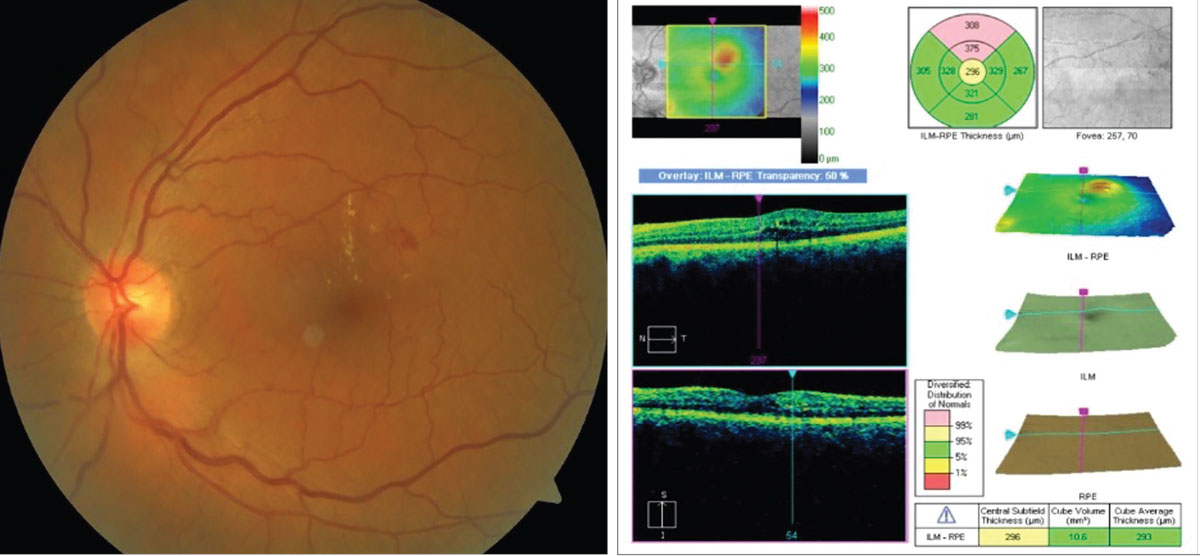
For nearly 40 years, the diagnosis, management and treatment of diabetic retinopathy (DR) and diabetic macular edema (DME) has been dominated by the landmark Early Treatment of Diabetic Retinopathy Study (ETDRS).1 This famous trial was a randomized controlled study that enrolled 3,711 patients. The ETDRS originally sought to determine if and when laser photocoagulation is effective in both of these conditions. An example of an eye with DME is shown in Figure 1.
Amongst its many seminal contributions, the ETDRS developed the criteria for clinically significant and non–clinically significant diabetic retinopathy (Table 1). The study demonstrated that macular photocoagulation could decrease the risk of moderate visual loss from clinically significant macular edema (CSME). The ETDRS also resulted in a DR grading scale that linked retinal findings to the risk of developing proliferative diabetic retinopathy (PDR). While the ETDRS continues to be impactful in eye care, since its publication there have been significant newer developments in diagnostic technology (OCT and angiography) and treatments (e.g., intravitreal anti-VEGF agents) that are now routinely applied to diabetic retinopathy. These technologies and treatments have necessitated the development of new assessment and management paradigms for DR and DME.
This article concludes a four-part series on the role of medical research in eye care with an in-depth look at the recent history of diabetic eye disease as advanced by one prominent network of researchers.
 |
|
Fig. 1. Example of a patient with diabetic macular edema. The OCT results (right) demonstrate an area of retinal thickening superior to the central subfield and corresponding to the area of hard exudates on the left. Photos: J.P. Maszczak, OD. Click image to enlarge. |
A Concerted Effort
In 2002 the Diabetic Retinopathy Clinical Research Network, now called the DRCR Retina Network (or DRCR for short), was formed with the goal of supporting multicenter clinical research initiatives in diabetes and other retinal disorders. To date, DRCR consists of over 160 research sites and is affiliated with over 500 physicians throughout the United States and Canada.2 The DRCR Retina Network has published studies evaluating the outcomes of treatment of DR and DME with various modalities, including retinal laser photocoagulation, various intravitreal anti-VEGF agents, intravitreal steroids and nonsteroidal anti-inflammatory drugs.
With the additional imaging detail provided by OCT, new classifications and management protocols for DME have emerged from these studies. While the terms clinically significant and non–clinically significant are still broadly used, now macular edema is often described as either center-involved (CI) or non–center-involved (NCI) macular edema (Table 1).
 |
| Click image to enlarge. |
DRCR has grouped its studies under various protocols, each of which focuses on a different clinical question.2 In this article, we’ll look at some of the results from protocols that we feel have been particularly impactful for eyecare practice. A list of the published articles derived from all of the DRCR protocols as well as ongoing studies can be found here.
While many of the protocols developed by the DRCR Retina Network have focused on the treatment of DME and PDR, and thus are perhaps more applicable to our colleagues in ophthalmology, here we will emphasize protocols that can influence how optometrists manage our patients with DME. Of significant note is Protocol V, which concerns the management of patients with center-involving DME and good visual acuity. Special emphasis will be placed on this protocol. Interesting findings from some of the other protocols are shown in Table 2. Included in this table are the years in which these results were published.
 |
| Click image to enlarge. |
Protocol V
As mentioned above, Protocol V (Treatment for CI-DME with Very Good VA Study) from the DRCR examined the eyes of patients with diabetes with DME and good visual acuity. The studies under this protocol were unique in that previous research on DME management had focused on patients with visual acuity worse than a particular threshold. For example, Protocols I and T of the DRCR enrolled patients with an upper threshold of 78 ETDRS letters or Snellen equivalent visual acuities of 20/32 or worse.20
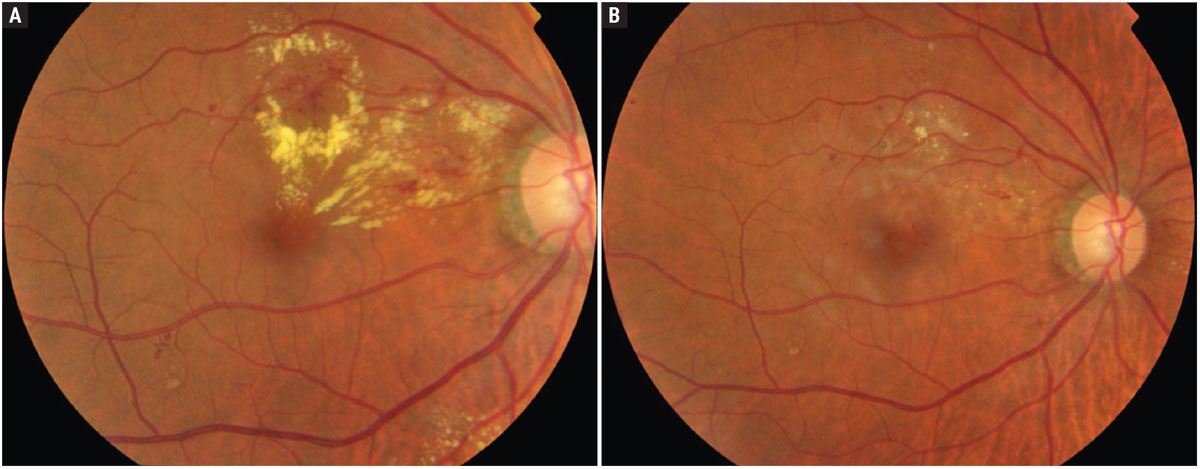
In 2019, Baker and colleagues published a seminal paper associated with Protocol V.21 Seven hundred and two adult individuals with type 1 or type 2 diabetes participated. Six hundred and twenty of these individuals completed the final two-year visit. Participants had been diagnosed with center-involved DME (thickening of the central macular subfield). Visual acuity in the eye under study had to demonstrate a Snellen equivalent visual acuity of 20/25 or better (at least 79 ETDRS letters). For the study eyes, mean baseline visual acuity letter score was 85.2 ±3.7 ETDRS letters. The mean hemoglobin A1c of the participants in all of the treatment groups was 7.6, more than 60% of the study eyes had moderate or better diabetic retinopathy, and the mean central subfield thickness was 311 ±57µm. Participants were initially randomized such that they received either aflibercept (2.0mg) injections (an injection at baseline and then “up to every four weeks as needed”) or focal/grid laser photocoagulation (Figure 2); a control group was observed.
 |
|
Fig. 2. Clinically significant macular edema before (A) and after (B) focal laser photocoagulation. From the publication of ETDRS until the advent of anti-VEGF therapies, this approach was routinely undertaken. Photos: Srinivasan Sanjay, MD. (Images used under Creative Commons 4.0 license. Original citation: Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diab Res. April 23, 2015.) Click image to enlarge. |
Injections were continued in those eyes that initially received aflibercept if visual acuity increased or decreased by five or more letters or central subfield thickness increased or decreased by ≥10% compared to either of the last two office visits. In the group initially treated with aflibercept injections, there were additional criteria (based on both the measured values and the stability of visual acuity and central subfield thickness) that were used to determine whether injections could be deferred, whether the follow-up period could be increased to eight and then 16 weeks, and whether laser photocoagulation was added. Eyes in the laser photocoagulation group received laser treatment at baseline and were retreated at 13 weeks if necessary. Eyes were re-examined at eight and 16 weeks in the photocoagulation and initial observation groups, and then every 16 weeks unless acuity declined or central subfield thickness increased. Eyes in the laser photocoagulation and observation groups received aflibercept injections if the visual acuity decreased from baseline by 10 or more letters at one visit, or by five to nine letters at two consecutive visits. In the two-year study period, 34% of eyes in the initial observation group and 25% of eyes in the laser photocoagulation group received “rescue” injections.
The primary outcome measure in the study was the number of eyes in which there was a decrease of at least five letters of visual acuity at two years, as this was considered to be a clinically meaningful loss of acuity. There was no significant difference at two years in the percentage of eyes with a five-letter acuity loss between the three treatment groups: aflibercept initially (16%), laser photocoagulation initially (17%) and observation initially (19%).
Additional analyses of these data in two other papers showed that there was no difference between the treatment groups in low-contrast visual acuity at two years and that there may be better cost savings on a “societal level” for patients with DME and good visual acuity if these patients are initially treated with laser photocoagulation or observation rather than aflibercept injections.22,23 The results from the primary outcome measure in the study of Baker and colleagues suggest that, initially, close observation of patients with CI-DME could be reasonable. However, as with most studies, there are a number of factors that must be considered in applying these results to clinical patients.
First, Baker and colleagues reported secondary outcome measures that were found to be significantly different between the different treatment groups. For example, the percentage of eyes with visual acuity of 20/20 or better at two years was 77% in the aflibercept group, 71% in the laser photocoagulation group, and 66% in the initial observation group.21 These percentages were compared and the difference between the aflibercept and the initial observation group was found to be statistically significant. This was the only significant difference for these visual acuity comparisons.
In another, prespecified analysis, the mean change in visual acuity letter score at two years was compared for the different treatment groups. These changes were +1.5 ±4.0 for the aflibercept group, 0.0 ±3.9 for the photocoagulation group and -0.4 ±4.2 for the observation group. Although these are relatively small differences, the value for visual acuity change for the aflibercept group was significantly different from the values for the other two groups. Clearly, the conclusion that the visual outcome was the same in all treatment groups requires some nuanced consideration.
In addition, each group in the study of Baker and colleagues had reasonably good HbA1c (median 7.6 in all three groups).20,21 While the relationship between blood sugar control and vision in patients with CI-DME and good vision is not clear, a positive correlation between persistent CSME and hemoglobin A1c has been reported, as has increased risk of CSME in patients with HbA1c values ≥8%.24,25 Further, the eyes in the study by Baker and colleagues had mean central subfield thicknesses (306 ±55µm in the aflibercept group, 314 ±52µm in the laser photocoagulation group and 314 ±64µm in the observation group) that, as pointed out by Wykoff, were much lower than the mean central subfield thickness of eyes in “most Phase III DME trials.”20 In these latter trials, central subfield thicknesses were in the 400s and 500s.
The significance of the central subfield thickness was examined in a study by Glassman and colleagues.26 In a secondary analysis of those data from the Baker study, Glassman et al. looked at factors correlated with the likelihood that eyes in the observation group required aflibercept injections. An eye was twice as likely to receive aflibercept injections if the central subfield thickness was at least 300µm or if the eye had moderately severe diabetic retinopathy (ETDRS Diabetic Retinopathy Severity Scale of 47 or higher), or if the non–study eye was treated for DME within four months of baseline.
Along with these aforementioned factors, there are other individual features that could influence the initial management of patients with CI-DME and visual acuity of 20/25 or better. Lane pointed out that issues around compliance with follow-up appointments could play a role in determining which treatment might be selected for a patient.27 In Baker and colleagues’ original study, the participants in the aflibercept group had a median number of 18 office visits over two years, while the median number of office visits was 11 for the laser photocoagulation group and 12 for the observation group. This suggests that some patients who may have difficulty making it to office visits might be best managed initially with laser photocoagulation or observation.
Cost might be a factor for individual patients as well. Hutton and colleagues demonstrated that there may be better cost savings if patients with CI-DME and good visual acuity are treated initially with laser photocoagulation rather than aflibercept.23 However, it should be noted that in the original Baker study, the median number of injections was nine in the observation group, eight in the aflibercept group and seven for the laser photocoagulation group, so costs related to intravitreal injections may be less of a factor than expected.
Lastly, a retrospective study by Busch and colleagues demonstrated that in a group of patients with good vision (≤0.1 logMAR or ≥20/25 Snellen) and CI-DME who were initially untreated, these patients were more likely to demonstrate significant decreases in visual acuity over a 12-month period in the presence of baseline OCT markers, including hyperreflective foci (HRF), disorganization of inner retinal layers (DRIL) and ellipsoid zone (EZ) disruption.28 See Figure 3 for examples of DRIL and HRF.
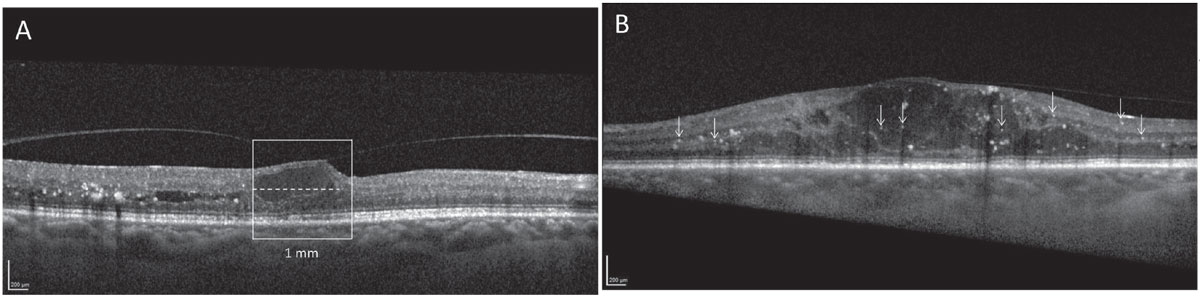 |
|
Fig. 3. Contemporary thinking on DME is such that “watchful waiting” may be appropriate in cases with good visual acuity. However, if inflammatory biomarkers such as (A) disorganization of retinal inner layers or (B) retinal hyperreflective foci are present, the patient may be suitable for referral to a retina specialist even in the absence of visual changes. Photos: Xinyi Chen, MD. (Images used under Creative Commons 4.0 license. Original citation: Chen X, Yang W, Fong A, et al. Sex differences in inflammation-related biomarkers detected with optical coherence tomography in patients with diabetic macular edema. Ophthalmol Sci. July 18, 2024.) Click image to enlarge. |
Taken together, all of these findings suggest that the initial approach in managing patients with CI-DME and good visual acuity should be tailored to the individual. The patient’s hemoglobin A1c level, central subfield thickness, level of diabetic retinopathy, history of treatment or lack thereof for DME in the fellow eye, OCT biomarkers (e.g., the presence of HRF, DRIL and EZ disruption), challenges in making it to scheduled office visits and perhaps cost considerations should all be factored in to the decision regarding the initial management plan.
The results of other studies with similar aims to that of Baker et al. must also be considered in determining how to manage patients with diabetes who have CI-DME and good visual acuity. In a study separate from those of the DRCR, Busch and colleagues performed a retrospective record review that included 249 eyes of 210 patients.29 Patients in the study were diabetes patients with CI-DME who had good visual acuity (≤0.1 logMAR or ≥20/25 Snellen). Some patients were treated at baseline while others were treated at some point over the 12-month period under study (various treatments were represented). Other patients were not treated.
At 12 months, most of the patients (58.1% in the treated group and 73.4% in the non-treated group) were found to have either gained visual acuity or to have lost less than five letters of acuity. However, if the visual acuity in the non-treated eyes decreased by five or more letters within six months, then the visual outcome for eyes was worse if no treatment was applied compared to cases where treatment occurred. The conclusion of this study was similar to that of Baker et al. in that close observation of patients with DME and good visual acuity is reasonable until the visual acuity drops by one line.
On the other hand, a non-DRCR study by Gabrielle and colleagues resulted in a slightly different conclusion from the work cited above.30 In a retrospective study, these investigators looked at data from patients with what was termed clinically significant diabetic macular edema, defined as DME within 500µm of the foveal center or at least one disc diameter of swelling, any part of which was within one disc diameter of the foveal center. Patients were required to have good visual acuity (≥79 letters read on a logMAR chart or 20/25 Snellen equivalent). Eyes were placed in the “initially treated” group if they received any type of treatment at baseline (e.g., anti-VEGF injection, steroid implant, macular laser photocoagulation). Eyes were placed in the “initially untreated” group if they were observed for at least four months after the baseline visit. Eyes in the “initially untreated” group were in some cases treated (66% received at least one intravitreal injection, 20% received macular laser photocoagulation, and 13% received at least one intravitreal injection and macular laser).
The primary outcome measure was the proportion of eyes with visual acuity loss of five or more letters at 24 months. The number of eyes that lost five or more letters at 24 months was 65% in the initially untreated group and 42% in the initially treated group. While this difference was not statistically significant and was therefore consistent with the results of the study of Baker et al. from the DRCR, eyes in the initially untreated group had a greater likelihood of 10-letter and 15-letter visual acuity losses compared to the initially treated group. While there are myriad differences between this study and that of Baker and colleagues—including, for example, much less rigid management protocols and the inclusion of patients with CS-DME and not just CI-DME—this study could suggest that the results from Protocol V may not extend perfectly to everyday clinical practice.
Clinical Management of CI-DME Patients and Good Visual Acuity
The study from Baker and colleagues performed under Protocol V of the DRCR Retina Network was the first to examine the management of CI-DME in patients with good visual acuity. Baker et al. concluded from the primary outcome measure that there was no difference in visual outcome at two years whether the study eyes were initially treated with aflibercept, initially treated with macular laser photocoagulation or initially observed.
This study has been foundational in determining how patients with CI-DME and good visual acuity are managed. However, as detailed above, combining the results of the study of Baker and colleagues with the results of other studies has made it clear that the decision as to how to manage a patient with CI-DME and good visual acuity must be based on a number of individual factors.
After a dilated fundus examination and an OCT scan, and after consideration of all of the patient-related factors, such as the patient’s HbA1c and ability to comply with follow-up schedules, if the decision is made to monitor patients with CI-DME and good visual acuity (≥20/25 Snellen as measured while the patient views through the most up-to-date refraction in place), the follow-up period should be no longer than six weeks. In keeping with findings from the studies of Protocol V and from other studies, in patients who are initially observed, if the visual acuity at any follow-up decreases by five or more letters on an ETDRS chart or visual acuity is measured at ≤20/32 on a Snellen chart, or the central subfield thickness as assessed by OCT increases by ≥10% compared to the initial visit, then a referral to retinal ophthalmology within two to four weeks is warranted.31
A referral should also be strongly considered if, at follow-up visits, the dilated fundus examination suggests worsening retinopathy overall (especially if the diabetic retinopathy exceeds the moderate level at a study visit), or the patient reports an increase in their HbA1c to a value over 8% (assuming this value was under 8% initially), or the OCT scan shows significantly more HRF, DRIL and EZ disruption. Finally, if the patient can no longer return for regular follow-up visits, this could be the basis for an ophthalmological referral.
Summary
Studies on diabetic retinopathy from the DRCR Retina Network have contributed substantially to the evidence base upon which eyecare practitioners rely to determine how to best manage our diabetic patients with retinal complications. Each study answers some questions and raises others, so it is important for practitioners to keep up on new developments. Finally, there may be details in these studies beyond the conclusions of the primary analyses that can further guide or refine management protocols.
Dr. Fogt is a professor of optometry and vision sciences at The Ohio State University College of Optometry in Columbus, OH. He teaches the posterior segment disease course and a graduate course in eye movements. Dr. Fogt has no financial disclosures.
Dr. Coates is an assistant clinical professor of optometry and vision sciences at The Ohio State University College of Optometry. He also has no financial disclosures.
1. Relhan N, Flynn HW Jr. The early treatment diabetic retinopathy study historical review and relevance to today's management of diabetic macular edema. Curr Opin Ophthalmol. 2017;28(3):205-212. 2. JAEB Center for Health Research. DRCR Retinal Network – Public Site Home Page. https://public.jaeb.org/drcrnet/view/home_page. Accessed November 4, 2024. 3. Diabetic Retinopathy Clinical Research Network; Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525-536. 4. Browning DJ, Apte RS, Bressler SB, et al. Association of the extent of diabetic macular edema as assessed by optical coherence tomography with visual acuity and retinal outcome variables. Retina. 2009;29(3):300-305. 5. Silva PS, Liu D, Glassman AR, et al. Assessment of fluorescein angiography nonperfusion in eyes with diabetic retinopathy using ultrawide field retinal imaging. Retina. 2022;42(7):1302-1310. 6. Silva PS, Marcus DM, Liu D, et al. Association of ultra-widefield fluorescein angiography-identified retinal nonperfusion and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140(10):936-945. 7. Jhaveri CD, Glassman AR, Ferris FL 3rd, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387(8):692-703. 8. Bressler SB, Glassman AR, Almukhtar T, et al. Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57-68. 9. Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross JG, Glassman AR, Jampol, LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. 10. Bressler SB, Beaulieu WT, Glassman AR, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology. 2017;124(4):431-439. 11. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. 12. Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87-95. 13. Bressler SB, Beaulieu WT, Glassman AR, et al. Photocoagulation versus ranibizumab for proliferative diabetic retinopathy: should baseline characteristics affect choice of treatment? Retina. 2019;39(9):1646-1654. 14. Maguire MG, Liu D, Glassman AR, et al. Visual field changes over 5 years in patients treated with panretinal photocoagulation or ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2020;138(3):285-293. 15. Diabetic Retinopathy Clinical Research Network; Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. 16. Jampol LM, Glassman AR, Bressler NM, Wells JA, Ayala AR; Diabetic Retinopathy Clinical Research Network. Anti-vascular endothelial growth factor comparative effectiveness trial for diabetic macular edema: additional efficacy post hoc analyses of a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1429-1434. 17. Glassman AR, Wells JA 3rd, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020;127(9):1201-1210. 18. Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701-712. 19. Maturi RK, Glassman AR, Josic K, et al. Four-year visual outcomes in the Protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA. 2023;329(5):376-385. 20. Wykoff CC. Thresholds for initiating treatment of eyes with diabetic macular edema and good vision: consideration of DRCR.net Protocol V results. Ophthalmol Retina. 2019;3(11):917-919. 21. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. 22. Beaulieu WT. Low-contrast visual acuity in eyes with diabetic macular edema and good vision initially treated with aflibercept, laser, or observation: an ancillary study from DRCR Retina Network Protocol V. Invest Ophthalmol Vis Sci. 2020;61(7):4874. 23. Hutton DW, Glassman AR, Stein JD, Bressler NM, Sun JK; DRCR Retina Network. Costs of managing diabetic macular edema with good visual acuity with aflibercept, laser, or observation: DRCR Retina Network Protocol V. Am J Ophthalmol. 2021;230:297-302. 24. Do DV, Shah SM, Sung JU, Haller JA, Nguyen QD. Persistent diabetic macular edema is associated with elevated hemoglobin A1c. Am J Ophthalmol. 2005;139(4):620-623. 25. Chou TH, Wu PC, Kuo JZ, Lai CH, Kuo CN. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye (Lond). 2009;23(6):1360-1363. 26. Glassman AR, Baker CW, Beaulieu WT, et al. Assessment of the DRCR Retina Network approach to management with initial observation for eyes with center-involved diabetic macular edema and good visual acuity: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020;138(4):341-349. 27. Lane RG. Putting Protocol V into practice for patients with DME. Retinal Physician. 2022;19:35,36-39. 28. Busch C, Okada M, Zur D, et al. Baseline predictors for visual acuity loss during observation in diabetic macular oedema with good baseline visual acuity. Acta Ophthalmol. 2020;98(7):e801-e806. 29. Busch C, Fraser-Bell S, Zur D, et al. Real-world outcomes of observation and treatment in diabetic macular edema with very good visual acuity: the OBTAIN study. Acta Diabetol. 2019;56(7):777-784. 30. Gabrielle PH, Nguyen V, Bhandari S, et al. Initial observation or treatment for diabetic macular oedema with good visual acuity: two-year outcomes comparison in routine clinical practice: data from the Fight Retinal Blindness! Registry. Acta Ophthalmol. 2022;100(3):285-294. 31. American Optometric Association. Eye Care of the Patient with Diabetes Mellitus. 2nd ed. American Optometric Association; 2019. |


