Surgical Insights for ODsIn the December 2024 issue of Review of Optometry—the magazine's 31st annual surgery report—learn how to spot and manage cataract surgery complications, review the newest techniques and procedures in refractive surgery and discover how laser procedures like SLT, LPI and YAG capsulotomy help your patients in the long term. Check out the other articles featured in this issue: |
As the scope of optometric practice has expanded, much education and training has been dedicated to the skills necessary to perform these advanced procedures. As important as the how-to is in successfully managing a patient’s condition, however, a thorough understanding of when (and when not) to consider a procedure can’t be overstated. A laser procedure is a significant event for the patient and, as optometry’s involvement grows, we must strive to see it in the context of their long-term care.
Here, we will address indications and contraindications for the three most commonly performed laser procedures: selective laser trabeculoplasty (SLT), laser peripheral iridotomy (LPI) and YAG capsulotomy. This information is pertinent for both optometrists who comanage these procedures as well as the ones who are performing them.
SLT
This in-office medical procedure is employed to help lower the intraocular pressure (IOP). Although it is not fully understood, the theory behind SLT is that one of two things happen:
(1) SLT targets the pigmented chromophores in the trabecular meshwork. The laser emits small bursts of energy that stimulate the contraction of the cells, allowing the aqueous to drain more effectively and ultimately reducing IOP.
(2) SLT causes a biological response in which the macrophages clean out the collector channels, allowing for much easier outflow of aqueous. Due to the very low energy levels used, there is little to no permanent, visible change to the tissue, and thus SLT can be repeated multiple times. Many eyecare professionals now use SLT as a first-line therapy for treating glaucoma, choosing it over traditional pharmacological treatment with IOP-lowering medications, given new research implying more favorable long-term glaucoma outcomes with SLT.1
Indications
Consider SLT in the following patients:• As a primary treatment for patients with open-angle glaucoma (OAG), pseudoexfoliative (PE) or pigmentary glaucoma (PG).
• OAG/PE/PG patients not properly controlled by medical therapy.
• OAG/PE/PG patients that have shown poor compliance to medical treatment.
• OAG/PE/PG patients with allergic reactions and/or poor tolerance to topical medications.
• OAG/PE/PG patients in whom IOP remains above target and/or their disease is progressing.
• Low-tension glaucoma.
• Ocular hypertension.
• For newly diagnosed patients with OAG/PE/PG, this can be offered as a first-line defense instead of using topical therapy.
Contraindications
SLT is not generally recommended for patients with these types of glaucoma:• inflammatory
• neovascular
• traumatic
• congenital
• angle-closure glaucoma
• advanced glaucoma
The procedure is also not recommended for glaucoma patients in whom there is poor visualization of the trabecular meshwork secondary to anatomic angle status and/or corneal opacity. Also, do not consider this for patients who cannot sit still or hold their head in position in the slit lamp for the procedure.
If at any time there is a concern that performing SLT would be risking the patients overall health of the eye, then the procedure should not be performed. Careful screening and consideration of the patient's health and ability to keep their head still is recommended.
 |
|
360° SLT is largely the standard for POAG, ocular hypertension and low-tension glaucoma. Photo credit: Nate Lighthizer, OD, and Komal Patel, OD. Click image to enlarge. |
What glaucoma specialists would like us to know about SLT complications
• Occasionally, there may be some mild soreness, redness or blurred vision in the immediate postoperative period. This may be caused by the SLT gonio lens, its coupling fluid, induced iritis and/or an IOP spike, and may be prophylactically or secondarily treated with a topical nonsteroidal anti-inflammatory or a topical corticosteroid several times per day for two to seven days post-procedure.2• A short-term IOP spike after SLT is not uncommon, is usually temporary and may be prophylactically and/or secondarily treated with a topical α agonist (either apraclonidine 0.5% or brimonidine 0.2%).
• Much less common with SLT vs. its predecessor argon laser trabeculoplasty, the incidence of peripheral anterior synechiae is very low (0% to 2.86%), but has been reported to occur in some patients after multiple SLT treatments.3
• Corneal edema is also possible post-SLT, with several potential etiologies to consider:
– Excessive digital pressure applied to the cornea from the gonio lens used in the procedure.
– HSV reactivation. While rare, the proinflammatory cascade following SLT may reactivate the virus in some patients, in which case treatment is with the usual topical and/or oral antiviral medications.4
Hyphema is an exceedingly rare finding with SLT but, if noted, treatment protocols are consistent with other hyphema etiologies, including topical medications and keeping the head elevated until the blood resolves from within the anterior chamber.
Post-op and Follow-up Care
The IOP should be checked approximately 30 to 60 minutes post-procedure to monitor for an IOP spike, and if present, ocular hypotensive medications may be prescribed. The patient is then examined again in two to six weeks.5 Depending on the IOP response, the fellow eye may be scheduled for SLT and/or normal glaucoma follow up protocols can then be resumed.
Coming soon in the US is direct SLT (Alcon). This device uses eye-tracking technology to ensure an accurate, automated treatment delivery through the limbus—eliminating the need for a gonio lens or manual aiming.6
LPI
This surgical procedure creates a small, full-thickness opening through the iris and is usually performed superiorly, between 11 o’clock and one o’clock, allowing for the upper lid to cover the opening in order to potentially reduce visual dysphotopsias, which may occur. Some studies have advocated for a three o’clock or nine o’clock LPI location to minimize dysphotopsias created by the tear prism at the superior lid margin, but there is no clear consensus on the best location to perform LPI. The purpose of the procedure is to allow aqueous from the posterior chamber to flow through the LPI opening into the anterior chamber and then into the trabecular meshwork in an unrestricted fashion to reduce the risk of angle closure. LPI may be performed with a YAG laser and/or an argon laser.
Indications
LPI is most commonly used in patients who have anatomically narrow angles, narrow angle glaucoma or in cases of pupillary block.7 In rare cases, LPI may be performed prior to surgical insertion of an implantable contact lens for refractive correction. In the scenarios listed above, the end goal is to help maintain normal IOP for the patient.
 |
|
LPI may provide a solution for those at high risk of developing primary angle closure. Photo credit: Joseph Shetler OD, and Nate Lighthizer, OD. Click image to enlarge. |
Contraindications
As with any surgical procedure, caution should always be taken when making the decision to perform an LPI. Active intraocular inflammation should be treated prior to performing any laser procedure; if the inflammation cannot be controlled or resolved, the LPI should not be performed. In patients with phacomorphic narrowing of the angle, a strong consideration should be given to cataract extraction instead of LPI.
What glaucoma specialists would like us to know about LPI
• Careful and thorough discussion with the patient regarding the procedure should always occur as a condition of informed consent. The patient should demonstrate understanding of the purpose of LPI, alternative options (including the aforementioned cataract extraction in phacomorphic cases or monitoring) and potential risks. It’s imperative to provide specific written postoperative instructions, including medication administration regimens, directions for contacting the provider in the event of a complication and when scheduled follow-up should occur.
• Here are some potential postoperative complications to be aware of:
– IOP spike. There may be a temporary increase in IOP, in which case an ocular hypotensive medication should be prescribed pending post-op follow-up in a few weeks. In the absence of indications to the contrary, a topical alpha-2 agonist is the medication of choice to reduce IOP due to the mild miosis it induces while also reducing aqueous production and enhancing aqueous outflow. This medication may be used immediately prior to and after surgery to blunt any potential IOP spike, then may be prescribed twice a day afterwards if needed.
– Iritis. The laser energy used to create the LPI opening may cause the ocular tissues to become inflamed. To reduce this risk and minimize loss of patency, a topical corticosteroid is usually prescribed QID for one to two weeks post-LPI.
– Hyphema. If the YAG laser strikes an iris blood vessel, it will bleed and will require tamponade of the anterior chamber via the laser LPI lens to stanch the bleeding. Once hemostasis is achieved, a microhyphema or a larger hyphema may persist, in which case typical standard protocols for hyphema management are initiated, including limiting physical activities and sleeping with head elevated until the blood has resorbed.
– Mild discomfort. This is not uncommon after LPI, and can be treated with acetaminophen; nonsteroidal anti-inflammatories such as aspirin and ibuprofen are relatively contraindicated if hyphema is present due to their anticoagulant effects.
– Photophobia/blurred vision. A small number of patients may temporarily experience these symptoms secondary to the iris debris released when creating the LPI, and/or from the laser lens and coupling fluid used during the procedure. These symptoms may be treated with topical corticosteroids several times a day pending follow-up. The patient may also wear tinted lenses or sunglasses to enhance comfort.
Postoperative and follow-up care
After the LPI is performed, it is recommended to check IOP within 30 to 60 minutes of surgery, then again at the post-op follow-up in one to two weeks.8 Gonioscopy is another critical procedure to perform, allowing for a better visualization and to check for patency of the PI. If the entirety of the angle is deepened, the LPI remains patent and IOP is normal, then regular exam intervals may be resumed.
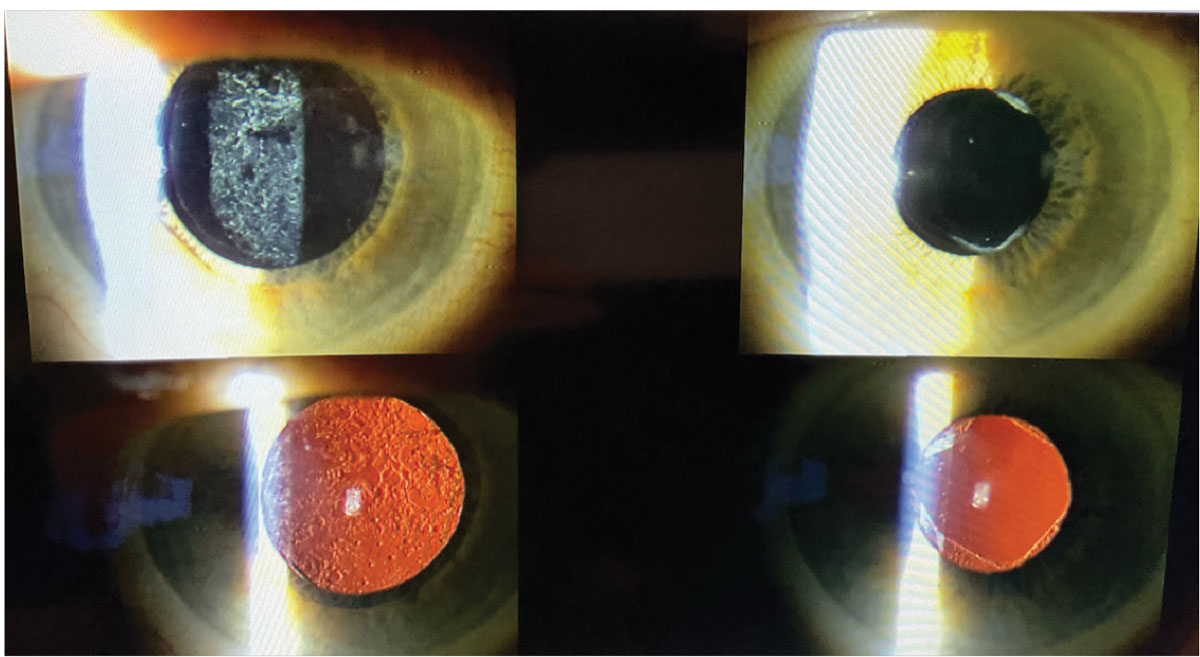 |
|
The photos on the left show the pre-op appearance of a patient with grade 3 posterior capsule opacification. The right two photos reveal the post-op appearance after a YAG capsulotomy was performed by an optometrist, showing perfect clearing of the posterior capsule. Photo credit: Nate Lighthizer, OD. Click image to enlarge. |
YAG Capsulotomy
This procedure uses a yttrium-aluminum-garnet (YAG) laser to treat posterior capsule opacification, a frequent complication that can occur following cataract surgery. Posterior capsular opacification, sometimes referred to as “secondary cataract,” develops when the posterior part of the lens capsule becomes cloudy, leading to a gradual decline in visual acuity. YAG capsulotomy uses a laser to create an opening in the cloudy capsule, restoring clear vision without the need for invasive surgery. This procedure is typically safe and effective, with minimal downtime, and is a vital part of post-cataract care.
Indications
A capsulotomy is indicated “if they see it and you see it,” meaning if the eyecare provider sees a cloudy posterior capsule and the patient has a visual complaint consistent with the level of posterior opacification present. Keep in mind the same requirements for medical necessity for cataract surgery also exist for YAG capsulotomy if the procedure is being billed to the patient’s medical insurance. The patient’s medical insurance guidelines for YAG capsulotomy should be reviewed, but in general there must be a documented effect on daily living, a two-line reduction in best-corrected acuity attributable to the opacity and/or a brightness acuity test that demonstrates a two-line reduction in acuity from glare.
If patients are symptomatic but not eligible for surgery under their medical insurance, they can be given the option of paying your usual and customary fee out-of-pocket. If a patient reports their vision fluctuates throughout the day, ocular surface disease or something else is most likely the cause, as the cloudy capsule obviously does not fluctuate. If the patient does not have a visual complaint that matches the level of opacification and/or clinically significant opacification is not observed, a capsulotomy should not be performed.
It is not unusual for patients who see 20/20 to complain that their vision has deteriorated right after cataract surgery and who present with mild posterior capsular opacification. Then, after capsulotomy is performed—while the patient’s acuity remains 20/20—they often report a marked subjective improvement in vision.
There are some unique situations where a capsulotomy may still be indicated even if the capsule itself is clear. One of these is when capsular contraction causes folds in the capsule, creating visual distortion and dysphotopsia. This may involve either the anterior or posterior capsule.
Anterior capsular phimosis results from fibrosis and contraction of the anterior capsule following cataract surgery capsulorhexis. If this contraction impinges on the visual axis and causes decreased acuity or other visual complaints, a capsulotomy may be indicated. In anterior capsular contraction syndrome, phimosis of the anterior capsule may cause the intraocular lens (IOL) to flex, in which case a capsulotomy is necessary to reduce the tension on the IOL.
With posterior capsular contraction syndrome, a Z formation of the IOL may sometimes occur. In this situation, one lens haptic is bent forward and the other is bent backwards. This is seen with plate haptic IOLs and accommodating IOLs (Crystalens, Bausch + Lomb), resulting in myopic and astigmatic shifts. It is standard practice now to perform a capsulotomy on Crystalens patients as soon as any lenticular astigmatism or fibrosis is observed. If the capsulotomy does not resolve the issues, alternative surgical correction may be necessary.
Occasionally, a YAG capsulotomy will be performed on a clear capsule in patients prior to corneal refractive surgery. This intervention is done as a prophylactic measure to reduce the risk of future lens flexure and refractive changes from capsular contraction syndromes.
 |
Posterior capsular distension syndrome before and after Nd:YAG capsulotomy. Photo credit: Alia Cappellani, OD, and Sophia Leung, OD. Click image to enlarge. |
Relative Contraindications
An open posterior capsule makes managing the vitreous more challenging if an IOL exchange is ever needed. If there is a possibility of an IOL exchange, the cataract surgeon should be consulted prior to performing a capsulotomy.
Caution is also advised any time visibility of the capsule is hindered due to corneal pathology. This visual obscuration could result from scars, epithelial basement membrane dystrophy (EBMD), radial keratotomy, Fuchs’ dystrophy or edema. Visualization may be improved with the use of a contact capsulotomy laser lens, which also has the advantage of improved focusing, enhanced control of the eye and concentration of the laser beam. However, in cases of advanced EBMD there is a risk of corneal debridement/erosion if the laser lens adheres to the epithelium. A cornea that is not clear may also necessitate the use of a higher power setting, increasing the risk of complications such as IOP spike, cystoid macular edema (CME), inflammation and, in rare cases, retinal detachment.9
Stable fixation and head stability in the laser are also required to perform a capsulotomy. The use of a contact capsulotomy laser lens can aid in fixation, as it often suctions onto the eye with the coupling solution and may help temporarily reduce or eliminate blinking. Oral sedatives are sometimes used to reduce tremors and involuntary movements, and head straps may also aid with stability. Occasionally, despite the aforementioned aids, stability cannot be achieved. In these cases, surgical dissection under general anesthesia by a cataract or retina surgeon may be indicated.
The most germane complications associated with capsulotomy (as with most laser procedures) are inflammation, IOP spike and, in rare cases, retinal detachment. Therefore, any patient who has or is at increased risk for any of these conditions should be treated with caution.
Intraocular inflammation should be treated prior to a capsulotomy. This includes postoperative inflammation from the cataract surgery. It is generally advised to wait at least three months post-cataract surgery to perform a capsulotomy. This recommendation, while helping to ensure inflammation has completely resolved, is primarily to create a window to allow for IOL exchange if necessary. In addition to waiting a minimum of three months, there is some evidence that suggests that intraocular inflammation should be resolved for one month prior to performing the capsulotomy.10
Patients with new or worsening CME should not undergo YAG capsulotomy until the condition is resolved or, at a minimum, stabilized. For this reason, it is recommended that a macular OCT be obtained on all patients prior to a capsulotomy to screen for CME. Patients who have had a vitrectomy, are diabetic or who have an epiretinal membrane are at higher risk of developing CME and should also be treated with caution. One approach is to pre-treat these patients with a topical nonsteroidal anti-inflammatory one week prior to the procedure.3
Caution is also advised with patients who are at high risk of retinal detachment. These include patients whose axial length is greater than 24mm, who have coincident lattice degeneration or any other vitreoretinal pathology, have experienced intraoperative complications during cataract surgery or have a history of previous retinal detachment.11 If YAG capsulotomy is performed, these patients should be monitored carefully during the postoperative period.
In all cases where patients are at higher risk of complications, it is advised to use lower total energy and to perform a smaller capsulotomy.
Absolute Contraindications
These are rare and involve older IOL materials that are infrequently seen in today’s patients. Glass IOL implants are a contraindication due to the risk of lens fracture.12 Older silicone and hydrogel lens materials may form calcification and crystalline deposits on the lens surface. This is typically not resolved with a capsulotomy, so an IOL exchange is required.13,14
Postoperative and Follow-up Care
After YAG capsulotomy is performed, patients are often prescribed a topical corticosteroid four times a day for one week, and are seen again in two to four weeks, at which time manifest refraction is performed along with slit lamp and dilated fundus examination. The latter may be postponed a few weeks until after the fellow eye is treated if both eyes require posterior capsulotomy.
Takeaways
Now that you have a broader picture of how these procedures help your patients care over the long-term course, as well as the potential risks and complications each one presents, it’s time to take this knowledge and your lasers to the next level.
Dr. Johnson is currently a professor at Rocky Mountain University College of Optometry and senior director of The RMU Eye Institute. Dr. Johnson’s clinic interests include ocular disease, lasers, contact lenses and advanced optometric procedures. Previously, he was a faculty member at the Oklahoma College of Optometry where he taught courses in neuro-ophthalmic disorders, healthcare systems and epidemiology, as well as trained students in the clinic on lasers and advanced optometric procedures. Prior to his career in academia, Dr. Johnson worked in the refractive surgery industry and owned a private practice.
Dr. Cummings is founder and owner of the Eye Center of Ephraim in Ephraim, UT, and is also a faculty member and teaches at Rocky Mountain University School of Optometry. His clinical experience includes ocular disease, particularly diabetes, glaucoma, keratoconus, macular degeneration and cataracts. Dr. Cummings is a member of both the Utah and American Optometric Associations and is actively involved in bringing eye care to underserved countries such as the Dominican Republic and Mali, Africa.
1. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (LiGHT) trial. A multicentre, randomized controlled trial: design and methodology. Br J Ophthalmol. 2018;102(5):593-8. 2. Johnson PB, Katz LJ, Rhee DJ. Selective laser trabeculoplasty: predictive value of early intraocular pressure measurements for success at three months. Br J Ophthalmol. 2006;90(6):741-3. 3. Baser EF, Akbulut D. Significant peripheral anterior synechiae after repeat selective laser trabeculoplasty. Can J Ophthalmol. 2015;50(3):e36-8. 4. Shaw E, Gupta P. Laser trabeculoplasty. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2024. National Library of Medicine, March 10, 2023. 5. Getting Started with Primary SLT, Christine Leonard, Associate Editor, Review of Ophthalmology, June 15, 2021. 6. Alcon Completes Acquisition of BELKIN Vision, Expanding Glaucoma Portfolio with Direct Selective Laser Trabeculoplasty (DSLT) Device. July 2024. www.alcon.com/media-release/alcon-completes-acquisition-belkin-vision-expanding-glaucoma-portfolio-direct/. 6. Laser peripheral iridotomy. Wills Eye Hospital. www.willseye.org/laser-peripheral-iridotomy. Accessed November 20, 2024. 8. Jiang Y, Chang DS, Foster PJ, et al. Immediate changes in intraocular pressure after laser peripheral iridotomy in primary angle-closure suspects. Ophthalmology. 2011;119(2):283-8. 9. Bhargava R, Kumar P, Phogat H, Chaudhary K. Neodymium-yttrium aluminium garnet laser capsulotomy energy levels for posterior capsule opacification. J Ophthalmic Vis Res. 2015;10(1):37. 10. Parajuli A, Joshi P, Subedi P, Pradhan C. Effect of Nd:YAG laser posterior capsulotomy on intraocular pressure, refraction, anterior chamber depth, and macular thickness. Clin Ophthalmol. 2019;13:945-52. 11. Karahan E, Er D, Kaynak S. An overview of Nd:YAG laser capsulotomy. Med Hypothesis Discov Innov Ophthalmol. 2014;3(2):45-50. 12. Steinert R. Nd:YAG laser capsulotomy. American Academy of Ophthalmology. www.aao.org/munnerlyn-laser-surgery-center/ndyag-laser-posterior-capsulotomy-3. Published November 4, 2013. Accessed November 20, 2024. 13. Izak AM, Werner L, Pandey SK, et al. Calcification on the surface of the Bausch & Lomb Hydroview Intraocular Lens. Int Ophthalmol Clin. 2001;41(3):63-77. 14. Altaie R, Loane E, O’Sullivan K, Beatty S. Surgical and visual outcomes following exchange of opacified Hydroview(R) intraocular lenses. Br J Ophthalmol. 2007;91(3):299-302. |


